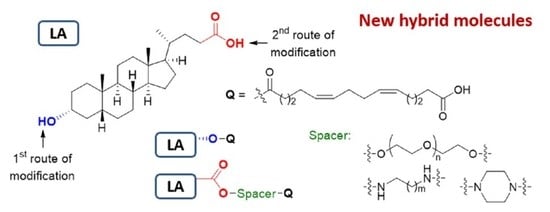
3.9. Chemical Experimental Data
1H NMR (CDCl
3, 400 MHz) and
13C NMR (CDCl
3, 100 MHz) spectral data and synthesis method for 2,2’-[(5
Z,9
Z)-tetradeca-5,9-diene-1,14-diylbis(oxy)]bistetrahydro-2H-pyran 3 and (5Z,9Z)-tetradeca-5.9-dienedioic acid 5 are described in the literature [
23].
3.9.1. General Procedure for the Synthesis of (5Z,9Z)-Tetradeca-5,9-diene-1,14-diol (4)
To a mixture of methanol (10 mL), CHCl3 (10 mL), and 1,14-tetrahydropyranyl-5Z,9Z-dien-1,14-diol 3 (0.39 g, 1.0 mmol), catalytic amounts of p-toluenesulfonic acid (p-TSA) were added, and the reaction mixture was stirred at 60 °C for 4 h. After this, the solvent was evaporated, and then the organic extract was washed with a saturated solution of sodium bicarbonate (3 × 25 mL). The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography using hexane/ethyl acetate (3:1) as the elution solvent to afford 0.52 g (76% yield) of diol 4.
(5Z,9Z)-Tetradeca-5,9-diene-1,14-diol (4)
Colorless oil, 0.17 g, 76% yield. IR (KBr) νmax 3006, 2924, 2852, 1657, 1466, 1408, 1384, 1214, 1165, 1100, 1034, 965, 911, 861, 755, 723, 665 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.42–5.34 (4H, m, H-5, H-6, H-9, H-10), 3.57 (4H, t, J = 6.5 Hz, H-1, H-14), 2.07–2.01 (8H, m, H-4, H-7, H-8, H-11), 1.58–1.52 (4H, m, H-2, H-13), 1.43–1.36 (4H, m, H-3, H-12); 13C NMR (CDCl3, 125 MHz) δ 129.9 (C-6, C-9), 129.5 (C-5, C-10), 62.6 (C-1, C-14), 32.3 (C-2, C-13), 27.4 (C-7, C-8), 26.9 (C-4, C-11), 25.8 (C-3, C-12).
3.9.2. Reaction of Methyl Ester of Lithocholic Acid (7) with (5Z,9Z)-Tetradeca-5.9-dienedioic Acid (5)
Lithocholic acid derivative
8 was synthesized according to a modified literature procedure [
26]. To a solution of methyl ester of lithocholic acid (0.39 g, 1.0 mmol) in CH
2Cl
2 (50 mL), (5
Z,9
Z)-tetradeca-5.9-dienedioic acid 4 (0.51 g, 2.0 mmol) was added, followed by EDC·HCl (0.48 g, 2.5 mmol) and DMAP (18 mg, 0.15 mmol) under argon. The mixture was stirred at room temperature for 12 h until the reaction was complete (TLC monitoring, hexane/ethyl acetate). The mixture was diluted with H
2O (10 mL) and the CH
2Cl
2 layer was separated, dried over MgSO
4, and concentrated. The crude product was purified by column chromatography (silica gel) using hexane/ethyl acetate (5:1) as the elution solvent to afford compound
8.
(5Z,9Z)-14-{[(3α,5β)-24-Methoxy-24-oxocholan-3-yl]oxy}-14-oxotetradeca-5,9-dienoic acid (8)
Colorless waxy solid, 0.42 g, 67% yield. [α]D21 + 30.3 (c 0.36, CHCl3); IR (KBr) νmax 2929, 2867, 1735, 1709, 1449, 1384, 1326, 1307, 1243, 1165, 1100, 1022, 802, 722 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.43–5.35 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.79–4.71 (1H, m, H-3), 3.68 (3H, s, O-CH3), 2.37 (2H, t, J = 7.5 Hz, H-2’), 2.35‒1.02 (28H, m), 2.29 (2H, t, J = 7.5 Hz, H-13’), 2.12–2.06 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.73–1.67 (4H, m, H-3’, H-12’), 0.94 (3H, s, H-19), 0.92 (3H, d, J = 6.4 Hz, H-21), 0.66 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 178.8 (C-1’), 174.9 (C-24), 173.5 (C-14’), 130.4, 130.1 (C-6’, C-9’), 129.1, 128.8 (C-5’, C-10’), 74.3 (C-3), 56.5 (C-14), 55.9 (C-17), 51.5 (O-CH3), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.4 (C-20), 35.0 (C-1), 34.6 (C-10), 34.2 (C-13’), 33.3 (С-2’), 32.3 (C-4), 31.1 (C-23), 31.0 (C-22), 28.2 (C-16), 27.4, 27.3 (C-7’, C-8’), 27.0 (C-6), 26.7 (C-2), 26.6 (C-11’), 26.5 (C-4’), 26.3 (C-7), 25.0 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C39H62O6: C, 74.72; H, 9.97; found C, 74.67; H, 9.92.
3.9.3. Reaction of 3α-Acetoxy-5β-cholan-24-oic Acid with (5Z,9Z)-Tetradeca-5,9-diene-1,14-diol (4)
Lithocholic acid derivative
10 was synthesized according to a modified literature procedure [
38]. To a solution of 3α-acetoxy-5β-cholan-24-oic acid (0.42 g, 1.0 mmol) in CH
2Cl
2 (50 mL), (5Z,9Z)-tetradeca-5,9-diene-1,14-diol (0.46 g, 2.0 mmol) was added, followed by EDC·HCl (0.48 g, 2.5 mmol) and DMAP (18 mg, 0.15 mmol) under argon. The mixture was stirred at room temperature for 12 h until the reaction was complete (TLC monitoring, hexane/ethyl acetate). The mixture was diluted with H
2O (10 mL) and the CH
2Cl
2 layer was separated, dried over MgSO
4, and concentrated. The crude product was purified by column chromatography (silica gel) using hexane/ethyl acetate (5:1) as the elution solvent to afford compound
10.
(5Z,9Z)-14-Hydroxytetradeca-5,9-dien-1-yl (3α,5β)-3-(acetyloxy)cholan-24-oate (10)
Colorless waxy solid, 0.44 g, 71% yield. [α]D19 + 25.0 (c 0.94, CHCl3); IR (KBr) νmax 2933, 2865, 1736, 1451, 1382, 1363, 1242, 1166, 1028, 911, 811, 756 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.40–5.31 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.73–4.66 (1H, m, H-3), 4.05 (2H, t, J = 6.8 Hz, H-1’), 3.62 (2H, t, J = 6.4 Hz, H-14’), 2.35‒1.00 (28H, m), 2.11–2.03 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.01 (3H, s, COCH3), 1.68–1.65 (2H, m, H-2’), 1.57–1.51 (2H, m, H-13’), 1.44–1.36 (4H, m, H-3’, H-12’), 0.92 (3H, s, H-19), 0.90 (3H, d, J = 6.4 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.4 (C-24), 170.6 (COCH3), 129.9, 129.7, 129.6, 129.4 (C-5’, C-6’, C-9’, C-10’), 74.4 (C-3), 64.2 (C-1’), 62.7 (C-14’), 56.5 (C-14), 55.9 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.3 (C-20), 35.0 (C-1), 34.6 (C-10), 32.4 (C-13’), 32.2 (C-4), 31.3 (C-23), 31.0 (C-22), 28.2 (C-2’, C-16), 27.4, 27.3 (C-7’, C-8’), 27.0 (C-2), 26.9, 26.8 (C-4’, C-11’), 26.6 (C-6), 26.3 (C-7), 25.9 (C-3’), 25.8 (C-12’), 24.2 (C-15), 23.3 (C-19), 21.4 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C40H66O5: C, 76.63; H, 10.61; found C, 76.51; H, 10.53.
3.9.4. Oxidation of the Esterification Product 10 with Jones Reagent
To a solution of compound 10 (0.44 g, 0.7 mmol) in acetone (25 mL) and CH2Cl2 (5 mL) at room temperature, Jones reagent (0.75 mL) was added dropwise. The reaction mixture was stirred at room temperature for 0.5 h, then quenched with water (10 mL), concentrated under reduced pressure to remove the excess of acetone and CH2Cl2, and the aqueous layer was extracted with ethyl acetate (3 × 20 mL). The organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by column chromatography using hexane/ethyl acetate (3:1) as the elution solvent to afford dienoic acid 11.
(5Z,9Z)-14-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]oxy}tetradeca-5,9-dienoic Acid (11)
Colorless waxy solid, 0.37 g, 83% yield. [α]D23 + 26.4 (c 0.66, CHCl3); IR (KBr) νmax 2931, 2866, 1736, 1709, 1450, 1382, 1363, 1242, 1167, 1096, 1027, 980, 803, 726 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.44–5.34 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.76–4.70 (1H, m, H-3), 4.07 (2H, t, J = 6.4 Hz, H-14’), 2.39–2.33 (2H, m, H-2’), 2.35‒1.02 (28H, m), 2.13–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.04 (3H, s, COCH3), 1.73–1.67 (2H, m, H-3’), 1.66–1.61 (2H, m, H-13’), 1.47–1.41 (2H, m, H-12’), 0.93 (3H, s, H-19), 0.92 (3H, d, J = 6.4 Hz, H-21), 0.65 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 179.2 (C-1’), 174.5 (C-24), 170.8 (COCH3), 130.4, 129.7 (C-6’, C-9’), 129.6, 128.8 (C-5’, C-10’), 74.4 (C-3), 64.3 (C-14’), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.4 (C-20), 35.0 (C-1), 34.6 (C-10), 33.4 (C-2’), 32.2 (C-4), 31.3 (C-23), 31.0 (C-22), 28.2 (C-13’, C-16), 27.3 (C-7’, C-8’), 27.0 (C-2), 26.8, 26.5 (C-4’, C-11’), 26.6 (C-6), 26.3 (C-7), 25.9 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.5 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C40H64O6: C, 74.96; H, 10.06; found C, 74.84; H, 9.99.
3.9.5. General Procedure for the Synthesis of Ethylene Glycol Derivatives of 3a-Acetoxy-5β-cholan-24-oic Acid and 3-Oxo-cholan-24-oic Acid 13a–d, 14a–d
To a solution of 3α-acetoxy-5β-cholan-24-oic acid 9 or 3-oxo-cholan-24-oic acid 12 (1.0 mmol) in CH2Cl2 (50 mL), ethylene glycol (2.5 mmol) was added, followed by EDC·HCl (0.58 g, 3.0 mmol) and DMAP (18 mg, 0.15 mmol) under argon. The mixture was stirred at room temperature for 16 h until the reaction was complete (TLC monitoring, hexane/ethyl acetate). The mixture was diluted with H2O (10 mL) and the CH2Cl2 layer was separated, dried over MgSO4, and concentrated. The crude product was isolated by column chromatography (silica gel) using hexane/ethyl acetate (1:1) as the elution solvent for 13a, 14a and hexane/ethyl acetate (1:3) for 13b–d, 14b–d.
2-Hydroxyethyl (3α,5β)-3-(Acetyloxy)cholan-24-oate (13a)
White crystals, 0.35 g, 76% yield. MP 130–132 °С, [α]D19 + 24.9 (c 1.05, CHCl3); IR (KBr) νmax 3016, 2938, 2868, 1736, 1449, 1381, 1363, 1243, 1170, 1086, 1027, 981, 949, 887, 756, 667, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.75–4.69 (1H, m, H-3), 4.23–4.19 (2H, m, СH2O), 3.84–3.80 (2H, m, СH2OH), 2.42‒1.02 (28H, m), 2.03 (3H, s, COCH3), 0.93 (3H, s, H-19), 0.92 (3H, d, J = 6.4 Hz, H-21), 0.65 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.7 (C-24), 170.7 (COCH3), 74.4 (C-3), 65.9 (СH2O), 61.2 (СH2OH), 56.5 (C-14), 55.9 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.4 (C-20), 35.0 (C-1), 34.6 (C-10), 32.2 (C-4), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.0 (C-6), 26.6 (C-2), 26.3 (C-7), 24.2 (C-15), 23.3 (C-19), 21.5 (COCH3), 20.8 (C-11), 18.2 (C-21), 12.0 (C-18); anal. calcd for C28H46O5: C, 72.69; H, 10.02; found C, 72.57; H, 9.96.
2-(2-Hydroxyethoxy)ethyl (3α,5β)-3-(Acetyloxy)cholan-24-oate (13b)
White crystals, 0.38 g, 74% yield. MP 72–74 °С, [α]D19 + 17.0 (c 0.82, CHCl3); IR (KBr) νmax 2938, 2868, 1736, 1449, 1381, 1363, 1243, 1171, 1132, 1066, 1028, 982, 949, 889, 755, 666, 616 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.63–4.57 (1H, m, H-3), 4.15–4.12 (2H, m, СH2O), 3.65–3.57 (4H, m, СH2O, СH2OH), 3.51–3.48 (2H, m, СH2O), 2.71 (1H, br s, OH), 2.32‒0.90 (28H, m), 1.91 (3H, s, COCH3), 0.83 (3H, s, H-19), 0.81 (3H, d, J = 6.4 Hz, H-21), 0.55 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.0 (C-24), 170.4 (COCH3), 74.2 (C-3), 72.4 (СH2O), 69.0 (СH2O), 63.2 (СH2O), 61.5 (СH2OH), 56.4 (C-14), 55.9 (C-17), 42.6 (C-13), 41.8 (C-5), 40.3 (C-9), 40.0 (C-12), 35.7 (C-8), 35.2 (C-20), 34.9 (C-1), 34.5 (C-10), 32.1 (C-4), 30.9 (C-23), 30.8 (C-22), 28.1 (C-16), 26.9 (C-6), 26.5 (C-2), 26.2 (C-7), 24.1 (C-15), 23.2 (C-19), 21.3 (COCH3), 20.7 (C-11), 18.2 (C-21), 11.9 (C-18); anal. calcd for C30H50O6: C, 71.11; H, 9.95; found C, 71.01; H, 9.86.
2-[2-(2-Hydroxyethoxy)ethoxy]ethyl (3α,5β)-3-(Acetyloxy)cholan-24-oate (13c)
Colorless waxy solid, 0.39 g, 71% yield. [α]D19 + 17.0 (c 0.82, CHCl3); IR (KBr) νmax 2938, 2867, 1735, 1635, 1450, 1384, 1243, 1171, 1124, 1066, 1026, 945, 880, 805, 722 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.66–4.60 (1H, m, H-3), 4.17–4.15 (2H, m, СH2O), 3.64–3.58 (8H, m, СH2O, СH2OH), 3.56–3.53 (2H, m, СH2O), 2.84 (1H, br s, OH), 2.35‒0.92 (28H, m), 1.95 (3H, s, COCH3), 0.86 (3H, s, H-19), 0.84 (3H, d, J = 6.5 Hz, H-21), 0.58 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.1 (C-24), 170.5 (COCH3), 74.3 (C-3), 72.5 (СH2O), 70.5, 70.3 (СH2O), 69.1 (СH2O), 63.2 (СH2O), 61.6 (СH2OH), 56.4 (C-14), 55.9 (C-17), 42.7 (C-13), 41.8 (C-5), 40.3 (C-9), 40.1 (C-12), 35.7 (C-8), 35.3 (C-20), 34.9 (C-1), 34.5 (C-10), 32.2 (C-4), 31.0 (C-23), 30.8 (C-22), 28.1 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 24.1 (C-15), 23.3 (C-19), 21.4 (COCH3), 20.8 (C-11), 18.2 (C-21), 11.9 (C-18); anal. calcd for C32H54O7: C, 69.78; H, 9.88; found C, 69.61; H, 9.85.
2-{2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy}ethyl (3α,5β)-3-(Acetyloxy)cholan-24-oate (13d)
Colorless waxy solid, 0.41 g, 70% yield. [α]D19 + 21.8 (c 0.64, CHCl3); IR (KBr) νmax 2938, 2867, 1735, 1636, 1450, 1381, 1243, 1171, 1124, 1066, 1028, 949, 914, 887, 845, 803, 733, 661, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.70–4.64 (1H, m, H-3), 4.20–4.18 (2H, m, СH2O), 3.71–3.59 (12H, m, СH2O, СH2OH), 3.57–3.55 (2H, m, СH2O), 2.87 (1H, br s, OH), 2.35‒0.95 (28H, m), 1.98 (3H, s, COCH3), 0.89 (3H, s, H-19), 0.87 (3H, d, J = 6.5 Hz, H-21), 0.60 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.2 (C-24), 170.6 (COCH3), 74.3 (C-3), 72.6 (СH2O), 70.6, 70.5, 70.3, (СH2O), 69.2 (СH2O), 63.3 (СH2O), 61.6 (СH2OH), 56.4 (C-14), 55.9 (C-17), 42.7 (C-13), 41.8 (C-5), 40.6 (C-9), 40.1 (C-12), 35.7 (C-8), 35.3 (C-20), 34.9 (C-1), 34.5 (C-10), 32.2 (C-4), 31.1 (C-23), 30.8 (C-22), 28.1 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 24.1 (C-15), 23.3 (C-19), 21.4 (COCH3), 20.8 (C-11), 18.2 (C-21), 12.0 (C-18); anal. calcd for C34H58O8: C, 68.65; H, 9.83; found C, 68.55; H, 9.74.
2-Hydroxyethyl (5β)-3-Oxocholan-24-oate (14a)
White crystals, 0.32 g, 77% yield. MP 134–136 °С, [α]D19 + 27.0 (c 0.79, CHCl3); IR (KBr) νmax 2936, 2865, 1734, 1713, 1446, 1381, 1299, 1249, 1179, 1084, 1035, 946, 887, 756, 667, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.16–4.10 (2H, m, СH2O), 3.77–3.73 (2H, m, СH2OH), 2.87 (1H, br s, OH), 2.70‒1.02 (28H, m), 0.96 (3H, s, H-19), 0.87 (3H, d, J = 6.5 Hz, H-21), 0.62 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 174.7 (C-24), 65.9 (СH2O), 60.9 (СH2OH), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.6 (C-9), 39.9 (C-12), 37.1 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.8 (C-10), 31.1 (C-23), 30.8 (C-22), 28.1 (C-16), 26.6 (C-6), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.1 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C26H42O4: C, 74.60; H, 10.11; found C, 74.51; H, 10.02.
2-(2-Hydroxyethoxy)ethyl (5β)-3-Oxocholan-24-oate (14b)
Colorless waxy solid, 0.35 g, 75% yield. [α]D19 + 20.0 (c 0.81, CHCl3); IR (KBr) νmax 2934, 2866, 1735, 1714, 1451, 1379, 1298, 1254, 1173, 1132, 1072, 966, 946, 889, 769, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.24–4.20 (2H, m, СH2O), 3.74–3.67 (4H, m, СH2O, СH2OH), 3.60–3.58 (2H, m, СH2O), 2.71‒1.03 (28H, m), 0.99 (3H, s, H-19), 0.90 (3H, d, J = 6.5 Hz, H-21), 0.66 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.4 (C-3), 174.2 (C-24), 72.3 (СH2O), 69.1 (СH2O), 63.3 (СH2O), 61.7 (СH2OH), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.7 (C-9), 40.0 (C-12), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.9 (C-10), 31.1 (C-23), 30.9 (C-22), 28.1 (C-16), 26.9 (C-6), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C28H46O5: C, 72.69; H, 10.02; found C, 72.56; H, 9.97.
2-[2-(2-Hydroxyethoxy)ethoxy]ethyl (5β)-3-Oxocholan-24-oateb (14c)
Colorless waxy solid, 0.37 g, 73% yield. [α]D17 + 20.5 (c 0.89, CHCl3); IR (KBr) νmax 2930, 2866, 1735, 1714, 1619, 1450, 1383, 1352, 1298, 1255, 1173, 1124, 1104, 1068, 945, 805, 530 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.20–4.18 (2H, m, СH2O), 3.69–3.61 (8H, m, СH2O, СH2OH), 3.56–3.54 (2H, m, СH2O), 2.72 (1H, br s, OH), 2.67‒1.03 (28H, m), 0.97 (3H, s, H-19), 0.87 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.2 (C-3), 174.1 (C-24), 72.5 (СH2O), 70.5, 70.3 (СH2O), 69.1 (СH2O), 63.2 (СH2O), 61.6 (СH2OH), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.6 (C-9), 39.9 (C-12), 37.1 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.8 (C-10), 31.0 (C-23), 30.8 (C-22), 28.1 (C-16), 26.6 (C-6), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.1 (C-11), 18.2 (C-21), 12.0 (C-18); anal. calcd for C30H50O6: C, 71.11; H, 9.95; found C, 71.06; H, 9.89.
2-{2-[2-(2-Hydroxyethoxy)ethoxy]ethoxy}ethyl (5β)-3-Oxocholan-24-oate (14d)
Сolorless waxy solid, 0.39 g, 70% yield. [α]D18 + 17.2 (c 0.99, CHCl3); IR (KBr) νmax 2931, 2866, 1734, 1714, 1619, 1451, 1383, 1298, 1259, 1173, 1125, 1105, 945, 887, 801, 531 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.19–4.16 (2H, m, СH2O), 3.70–3.60 (12H, m, СH2O, СH2OH), 3.56–3.54 (2H, m, СH2O), 2.83 (1H, br s, OH), 2.67‒1.03 (28H, m), 0.96 (3H, s, H-19), 0.87 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.2 (C-3), 174.1 (C-24), 72.5 (СH2O), 70.6, 70.5, 70.3, (СH2O), 69.2 (СH2O), 63.3 (СH2O), 61.6 (СH2OH), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.6 (C-9), 39.9 (C-12), 37.1 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.8 (C-10), 31.0 (C-23), 30.8 (C-22), 28.1 (C-16), 26.6 (C-6), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.1 (C-11), 18.2 (C-21), 12.0 (C-18); anal. calcd for C32H54O7: C, 69.78; H, 9.88; found C, 69.72; H, 9.83.
3.9.6. Reaction of Ethylene Glycol Derivatives of 3a-Acetoxy-5β-cholan-24-oic Acid and 3-Oxo-cholan-24-oic Acid 13a–d, 14a–d with (5Z,9Z)-Tetradeca-5,9-dienedioic Acid (5)
Lithocholic acid derivative
15a–
d and
16a–
d was synthesized according to a modified literature procedure [
26]. To a solution of ethylene glycol derivatives of 3α-acetoxy-5β-cholan-24-oic acid
13a–
d or 3-oxo-cholan-24-oic acid
14a–
d (1.0 mmol) in CH
2Cl
2 (50 mL), (5
Z,9
Z)-tetradeca-5.9-dienedioic acid (
5) (0.51 g, 2.0 mmol) was added, followed by EDC·HCl (0.48 g, 2.5 mmol) and DMAP (18 mg, 0.15 mmol) under argon. The mixture was stirred at room temperature for 16 h until the reaction was complete (TLC monitoring, hexane/ethyl acetate). The mixture was diluted with H
2O (10 mL) and the CH
2Cl
2 layer was separated, dried over MgSO
4, and concentrated. The crude product was purified by column chromatography (silica gel) using hexane/ethyl acetate (2:1) as the elution solvent to afford compounds
15a–
d or
16a–
d.
(5Z,9Z)-14-(2-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]oxy}ethoxy)-14-oxotetradeca-5,9-dienoic acid (15a)
Colorless waxy solid, 0.44 g, 64% yield. [α]D19 + 15.9 (c 0.76, CHCl3); IR (KBr) νmax 3065, 2937, 2865, 1947, 1735, 1679, 1450, 1385, 1244, 1141, 1105, 1026, 870, 803, 750, 690, 613 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.45–5.32 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.75–4.71 (1H, m, H-3), 4.41–4.39 (4H, m, СH2O), 2.40‒1.02 (28H, m), 2.39–2.33 (4H, m, H-2’, H-13’), 2.13–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.04 (3H, s, COCH3), 1.74–1.67 (4H, m, H-3’, H-12’), 0.94 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.66 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 178.7 (C-1’), 174.1 (C-24), 173.6 (C-14’), 170.7 (COCH3), 130.4, 130.3 (C-6’, C-9’), 128.9 (C-5’, C-10’), 74.4 (C-3), 62.1 (СH2O), 62.0 (СH2O), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.3 (C-20), 35.0 (C-1), 34.6 (C-10), 33.5 (C-13’), 33.2 (С-2’), 32.2 (C-4), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.3 (C-7’, C-8’), 27.0 (C-6), 26.6 (C-4’, C-11’), 26.5 (C-2), 26.3 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.5 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C42H66O8: C, 72.17; H, 9.52; found C, 72.11; H, 9.45.
(5Z,9Z)-14-[2-(2-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]oxy}ethoxy)ethoxy]-14-oxotetradeca-5,9-dienoic Acid (15b)
Colorless waxy solid, 0.46 g, 63% yield. [α]D19 + 20.0 (c 0.95, CHCl3); IR (KBr) νmax 3063, 2938, 2863, 1947, 1734, 1679, 1451, 1383, 1244, 1139, 1103, 1026, 870, 801, 750, 693, 614 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.42–5.31 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.72–4.68 (1H, m, H-3), 4.23–4.20 (4H, m, СH2O), 3.70–3.67 (4H, m, СH2O), 2.40‒0.95 (28H, m), 2.36–2.30 (4H, m, H-2’, H-13’), 2.10–2.03 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.01 (3H, s, COCH3), 1.73–1.66 (4H, m, H-3’, H-12’), 0.91 (3H, s, H-19), 0.89 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 178.8 (C-1’), 174.2 (C-24), 173.7 (C-14’), 170.7 (COCH3), 130.3, 130.2 (C-6’, C-9’), 128.9, 128.8 (C-5’, C-10’), 74.4 (C-3), 69.0 (СH2O), 63.2 (СH2O), 56.5 (C-14), 55.9 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.3 (C-20), 35.0 (C-1), 34.6 (C-10), 33.5 (C-13’), 33.3 (С-2’), 32.2 (C-4), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.3, 27.2 (C-7’, C-8’), 26.9 (C-6), 26.6 (C-11’), 26.5 (C-2, C-4’), 26.3 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.4 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C44H70O9: C, 71.12; H, 9.50; found C, 71.01; H, 9.43.
(5Z,9Z)-14-{2-[2-(2-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]oxy}ethoxy)ethoxy]ethoxy}-14-oxotetradeca-5,9-dienoic Acid (15c)
Colorless waxy solid, 0.48 g, 61% yield. [α]D19 + 15.5 (c 0.85, CHCl3); IR (KBr) νmax 3065, 2938, 2867, 1946, 1736, 1681, 1454, 1381, 1245, 1100, 1027, 981, 873, 802, 735, 698, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.45–5.30 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.77–4.72 (1H, m, H-3), 4.26–4.23 (4H, m, СH2O), 3.74–3.71 (4H, m, СH2O), 3.69–3.67 (4H, m, СH2O), 2.40‒1.00 (28H, m), 2.39–2.33 (4H, m, H-2’, H-13’), 2.13–2.06 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.04 (3H, s, COCH3), 1.74–1.67 (4H, m, H-3’, H-12’), 0.94 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.65 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 178.3 (C-1’), 174.3 (C-24), 173.8 (C-14’), 170.7 (COCH3), 130.4, 130.2 (C-6’, C-9’), 128.9 (C-5’, C-10’), 74.4 (C-3), 70.5 (СH2O), 69.3 (СH2O), 63.3 СH2O), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.4 (C-20), 35.0 (C-1), 34.6 (C-10), 33.6 (C-13’), 33.2 (С-2’), 32.2 (C-4), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.3 (C-7’, C-8’), 27.0 (C-6), 26.6 (C-4’, C-11’), 26.5 (C-2), 26.3 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.5 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C46H74O10: C, 70.20; H, 9.48; found C, 70.13; H, 9.43.
(5Z,9Z)-14-{2-[2-(2-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]oxy}ethoxy)ethoxy]ethoxy}-14-oxotetradeca-5,9-dienoic Acid (15d)
Colorless waxy solid, 0.50 g, 61% yield. [α]D19 + 14.0 (c 0.90, CHCl3); IR (KBr) νmax 2929, 2868, 1736, 1619, 1451, 1383, 1363, 1243, 1142, 1100, 1028, 802, 755, 673, 614 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.44–5.33 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.76–4.70 (1H, m, H-3), 4.26–4.23 (4H, m, СH2O), 3.74–3.71 (4H, m, СH2O), 3.69–3.67 (8H, m, СH2O), 2.40‒1.00 (28H, m), 2.39–2.33 (4H, m, H-2’, H-13’), 2.13–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.04 (3H, s, COCH3), 1.74–1.67 (4H, m, H-3’, H-12’), 0.94 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.65 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 177.3 (C-1’), 174.3 (C-24), 173.8 (C-14’), 170.7 (COCH3), 130.3, 130.2 (C-6’, C-9’), 128.9 (C-5’, C-10’), 74.4 (C-3), 70.6 (СH2O), 70.5 (СH2O), 69.32 (СH2O), 63.4 (СH2O), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 35.8 (C-8), 35.4 (C-20), 35.0 (C-1), 34.6 (C-10), 33.6 (C-13’), 33.1 (С-2’), 32.3 (C-4), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.3 (C-7’, C-8’), 27.0 (C-6), 26.6 (C-2, C-11’), 26.5 (C-4’), 26.3 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.5 (COCH3), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C48H78O11: C, 69.37; H, 9.46; found C, 69.30; H, 9.39.
(5Z,9Z)-14-(2-{[(5β)-3,24-Dioxocholan-24-yl]oxy}ethoxy)-14-oxotetradeca-5,9-dienoic Acid (16a)
Colorless waxy solid, 0.42 g, 65% yield. [α]D19 + 16.9 (c 0.73, CHCl3); IR (KBr) νmax 3063, 2938, 2865, 1953, 1729, 1679, 1443, 1385, 1239, 1125, 1105, 1011, 875, 803, 750, 690, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.44–5.33 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.31–4.26 (4H, m, СH2O), 2.73‒1.06 (28H, m), 2.39–2.32 (4H, m, H-2’, H-13’), 2.14–2.06 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.74–1.67 (4H, m, H-3’, H-12’), 1.02 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 178.3 (C-1’), 174.0 (C-24), 173.6 (C-14’), 130.3 (C-6’, C-9’), 128.9 (C-5’, C-10’), 62.1 (СH2O), 62.0 (СH2O), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.8 (C-13), 42.3 (C-4), 40.7 (C-9), 40.0 (C-12), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.9 (C-10), 33.5 (C-13’), 33.2 (С-2’), 31.1 (C-22), 30.9 (C-23), 28.2 (C-16), 27.3 (C-7’, C-8’), 26.6 (C-6), 26.5 (C-4’, C-11’), 25.8 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C40H62O7: C, 73.36; H, 9.54; found C, 73.29; H, 9.50.
(5Z,9Z)-14-[2-(2-{[(5β)-3,24-Dioxocholan-24-yl]oxy}ethoxy)ethoxy]-14-oxotetradeca-5,9-dienoic Acid (16b)
Colorless waxy solid, 0.44 g, 64% yield. [α]D19 + 17.9 (c 0.78, CHCl3); IR (KBr) νmax 3066, 2938, 2863, 1951, 1731, 1675, 1441, 1385, 1235, 1120, 1101, 1011, 875, 801, 750, 693, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.45–5.33 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.26–4.22 (4H, m, СH2O), 3.74–3.69 (4H, m, СH2O), 2.73‒1.06 (28H, m), 2.39–2.32 (4H, m, H-2’, H-13’), 2.14–2.06 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.74–1.66 (4H, m, H-3’, H-12’), 1.03 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 178.3 (C-1’), 174.2 (C-24), 173.8 (C-14’), 130.3, 130.2 (C-6’, C-9’), 128.9 (C-5’, C-10’), 69.1 (СH2O), 63.3 (СH2O), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.8 (C-13), 42.3 (C-4), 40.7 (C-9), 40.0 (C-12), 37.2 (C-2), 37.0 (C-1), 35.5 (C-8), 35.3 (C-20), 34.9 (C-10), 33.6 (C-13’), 33.2 (С-2’), 31.1 (C-22), 30.9 (C-23), 28.2 (C-16), 27.3 (C-7’, C-8’), 26.6 (C-6), 26.5 (C-4’, C-11’), 25.8 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C42H66O8: C, 72.17; H, 9.52; found C, 72.07; H, 9.45.
(5Z,9Z)-14-{2-[2-(2-{[(5β)-3,24-Dioxocholan-24-yl]oxy}ethoxy)ethoxy]ethoxy}-14-oxotetradeca-5,9-dienoic Acid (16c)
Colorless waxy solid, 0.45 g, 61% yield. [α]D18 + 12.5 (c 0.93, CHCl3); IR (KBr) νmax 2929, 2866, 1736, 1712, 1619, 1452, 1383, 1298, 1245, 1173, 1143, 1106, 1044, 965, 860, 731, 530 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.40–5.31 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.24–4.20 (4H, m, СH2O), 3.69–3.66 (4H, m, СH2O), 3.65–3.63 (4H, m, СH2O), 2.70‒1.04 (28H, m), 2.37–2.30 (4H, m, H-2’, H-13’), 2.13–2.03 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.71–1.65 (4H, m, H-3’, H-12’), 0.99 (3H, s, H-19), 0.90 (3H, d, J = 6.5 Hz, H-21), 0.66 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.7 (C-3), 178.5 (C-1’), 174.2 (C-24), 173.7 (C-14’), 130.3, 130.2 (C-6’, C-9’), 128.9, 128.8 (C-5’, C-10’), 70.5 (СH2O), 69.3 (СH2O), 63.3 (СH2O), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.7 (C-9), 40.0 (C-12), 37.1 (C-2), 36.9 (C-1), 35.5 (C-8), 35.3 (C-20), 34.9 (C-10), 33.6 (C-13’), 33.3 (С-2’), 31.1 (C-23), 30.8 (C-22), 28.1 (C-16), 27.3, 27.2 (C-7’, C-8’), 26.6 (C-6), 26.5 (C-4’, C-11’), 25.7 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C44H70O9: C, 71.12; H, 9.50; found C, 71.02; H, 9.44.
(5Z,9Z)-14-{2-[2-(2-{[(5β)-3,24-Dioxocholan-24-yl]oxy}ethoxy)ethoxy]ethoxy}-14-oxotetradeca-5,9-dienoic Acid (16d)
Colorless waxy solid, 0.48 g, 60% yield. [α]D18 + 10.4 (c 0.75, CHCl3); IR (KBr) νmax 2929, 2866, 1735, 1715, 1619, 1456, 1384, 1298, 1246, 1172, 1143, 1104, 1041, 947, 859, 825, 732, 530 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.45–5.34 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.26–4.23 (4H, m, СH2O), 3.74–3.69 (4H, m, СH2O), 3.68–3.66 (8H, m, СH2O), 2.74‒1.06 (28H, m), 2.40–2.33 (4H, m, H-2’, H-13’), 2.14–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.75–1.67 (4H, m, H-3’, H-12’), 1.03 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 177.4 (C-1’), 174.3 (C-24), 173.9 (C-14’), 130.3, 130.2 (C-6’, C-9’), 128.9 (C-5’, C-10’), 70.6 (СH2O), 70.5 (СH2O), 69.22 (СH2O), 63.4 (СH2O), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 40.7 (C-9), 40.1 (C-12), 37.2 (C-2), 37.0 (C-1), 35.5 (C-8), 35.3 (C-20), 34.9 (C-10), 33.6 (C-13’), 33.1 (С-2’), 31.1 (C-23), 30.9 (C-22), 28.2 (C-16), 27.4, 27.3 (C-7’, C-8’), 26.6 (C-6), 26.5 (C-4’, C-11’), 25.8 (C-7), 24.8 (C-12’), 24.6 (C-3’), 24.2 (C-15), 22.7 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C46H74O10: C, 70.20; H, 9.48; found C, 70.11; H, 9.41.
3.9.7. General Procedure for the Synthesis of BOC-Protected Diaminoalkane Derivatives of 3a-Acetoxy-5β-cholan-24-oic Acid and 3-Oxo-cholan-24-oic acid 19a–d, 20a–d
Oxalyl chloride (2 mL) was added to a solution of 9 or 12 (1.2 mmol) in CH2Cl2 (20 mL) and stirred at room temperature for 24 h excluding air humidity, affording the crude 24-acylchloride 17 or 18. After evaporation to dryness, another portion of CH2Cl2 (10 mL) was added, followed by diisopropylethylamine (0.5 mL, 3.0 mmol) and tert-butyl (2-aminoalcane)carbamate (1.8 mmol), stirred at room temperature for an additional 16 h, then evaporated, and the residue was purified by chromatography on silica gel using hexane/ethyl acetate (1:2) as the mobile phase, affording the product 19a–d or 20a–d.
(3α,5β)-24-({2-[(tert-Butoxycarbonyl)amino]ethyl}amino)-24-oxocholan-3-yl Acetate (19a)
White crystals, 0.60 g, 90% yield. MP 118–120 °С, [α]D19 + 32.4 (c 0.76, CHCl3); IR (KBr) νmax 2934, 2867, 1719, 1696, 1649, 1450, 1382, 1365, 1244, 1172, 1028, 755, 665, 610 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.62 (1H, br s, NH), 5.31 (1H, t, J = 6.5 Hz, NH), 4.69–4.63 (1H, m, H-3), 3.32–3.28 (2H, m, CH2N), 3.23–3.17 (2H, m, CH2N), 2.22‒0.95 (28H, m), 1.98 (3H, s, CH3CO), 1.39 (9H, s, CH3), 0.88 (3H, s, H-19), 0.86 (3H, d, J = 6.5 Hz, H-21), 0.59 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.4 (C-24), 170.6 (CH3CO), 156.9 (COO), 79.4 (C(CH3)3), 74.4 (C-3), 56.4 (C-14), 56.0 (C-17), 42.7 (C-13), 41.8 (C-5), 40.5 (CH2N), 40.4 (C-9), 40.3 (CH2N), 40.1 (C-12), 35.7 (C-8), 35.5 (C-20), 34.9 (C-1), 34.5 (C-10), 33.5 (C-23), 32.2 (C-4), 31.7 (C-22), 28.4 (CH3), 28.2 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 24.1 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); anal. calcd for C33H56N2O5: C, 70.68; H, 10.06; found C, 70.59; H, 9.98.
(3α,5β)-24-({4-[(tert-Butoxycarbonyl)amino]butyl}amino)-24-oxocholan-3-yl Acetate (19b)
White crystals, 0.61 g, 87% yield. MP 88–90 °С, [α]D21 + 26.9 (c 0.86, CHCl3); IR (KBr) νmax 2932, 2866, 1736, 1696, 1649, 1534, 1449, 1383, 1364, 1244, 1172, 1027, 754, 666, 608 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.21 (1H, br s, NH), 4.84 (1H, br s, NH), 4.66–4.60 (1H, m, H-3), 3.21–3.16 (2H, m, CH2N), 3.08–3.03 (2H, m, CH2N), 2.19‒0.93 (28H, m), 1.97 (3H, s, CH3CO), 1.48–1.44 (4H, m, CH2), 1.37 (9H, s, CH3), 0.86 (3H, s, H-19), 0.85 (3H, d, J = 6.5 Hz, H-21), 0.58 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 173.7 (C-24), 170.6 (CH3CO), 156.1 (COO), 79.4 (C(CH3)3), 74.3 (C-3), 56.4 (C-14), 56.0 (C-17), 42.7 (C-13), 41.8 (C-5), 40.4(C-9), 40.1 (C-12, CH2N), 39.0 (CH2N), 35.7 (C-8), 35.5 (C-20), 34.9 (C-1), 34.5 (C-10), 33.5 (C-23), 32.2 (C-4), 31.8 (C-22), 28.4 (CH3), 28.2 (C-16), 27.6 (CH2), 26.9 (C-6), 26.6 (C-2, CH2), 26.3 (C-7), 24.1 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C35H60N2O5: C, 71.39; H, 10.27; found C, 71.32; H, 10.21.
(3α,5β)-24-({6-[(tert-Butoxycarbonyl)amino]hexyl}amino)-24-oxocholan-3-yl Acetate (19c)
Colorless waxy solid, 0.61 g, 84% yield. [α]D21 + 26.9 (c 0.86, CHCl3); IR (KBr) νmax 2935, 2863, 1734, 1696, 1645, 1534, 1447, 1383, 1363, 1244, 1169, 1027, 753, 665, 608 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.84 (1H, br s, NH), 4.66–4.62 (1H, m, H-3), 4.64 (1H, br s, NH), 3.20–3.15 (2H, m, CH2N), 3.08–3.02 (2H, m, CH2N), 2.21‒0.95 (28H, m), 1.99 (3H, s, CH3CO), 1.48–1.40 (4H, m, CH2), 1.39 (9H, s, CH3), 1.31–1.25 (4H, m, CH2), 0.88 (3H, s, H-19), 0.87 (3H, d, J = 6.5 Hz, H-21), 0.59 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 173.6 (C-24), 170.6 (CH3CO), 156.1 (CO), 78.9 (C(CH3)3), 74.4 (C-3), 56.5 (C-14), 56.1 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4(C-9), 40.1 (C-12, CH2N), 39.1 (CH2N), 35.8 (C-8), 35.5 (C-20), 35.0 (C-1), 34.6 (C-10), 33.6 (C-23), 32.2 (C-4), 31.9 (C-22), 29.9 (CH2), 29.5 (CH2), 28.4 (CH3), 28.2 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 26.2 (CH2), 26.1 (CH2), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); anal. calcd for C37H64N2O5: C, 72.04; H, 10.46; found C, 71.96; H, 10.41.
tert-Butyl 4-[(3α,5β)-3(acetyloxy)-24-oxocholan-3-yl]piperazine-1-carboxylate Acetate (19d)
Colorless waxy solid, 0.58 g, 83% yield. [α]D21 + 25.0 (c 0.82, CHCl3); IR (KBr) νmax 2933, 2866, 1734, 1698, 1649, 1455, 1419, 1381, 1364, 1285, 1242, 1168, 1127, 1028, 997, 863, 754, 665, 614 cm−1; 1H NMR (CDCl3, 500 MHz) δ 4.72–4.66 (1H, m, H-3), 3.58–3.54 (2H, m, CH2N), 3.44–3.36 (6H, m, CH2N), 2.38‒0.96 (28H, m), 2.01 (3H, s, CH3CO), 1.45 (9H, s, CH3), 0.93 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 172.3 (C-24), 170.6 (CH3CO), 154.5 (COO), 80.2 (C(CH3)3), 74.3 (C-3), 56.5 (C-14), 56.1 (C-17), 45.4 (CH2N), 42.7 (C-13), 41.9 (C-5), 41.3 (CH2N), 40.4(C-9), 40.1 (C-12), 35.8 (C-8), 35.6 (C-20), 35.0 (C-1), 34.6 (C-10), 32.2 (C-4), 31.4 (C-23), 30.3 (C-22), 28.4 (CH3), 28.3 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.5 (C-21), 12.0 (C-18); anal. calcd for C35H58N2O5: C, 71.63; H, 9.96; found C, 71.58; H, 9.91.
tert-Butyl (2-{[(5β)-3,24-dioxocholan-24-yl)amino]ethyl}carbamate (20a)
White waxy solid, 0.55 g, 89% yield. [α]D17 + 19.0 (c 0.88, CHCl3); IR (KBr) νmax 2934, 2867, 1707, 1652, 1531, 1454, 1366, 1269, 1252, 1172, 1028, 754, 665, 531 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.36 (1H, br s, NH), 5.07 (1H, br s, NH), 3.37–3.33 (2H, m, CH2N), 3.29–3.26 (2H, m, CH2N), 2.72‒1.05 (28H, m), 1.44 (9H, s, CH3), 1.02 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.68 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.5 (C-3), 174.3 (C-24), 156.9 (COO), 79.6 (C(CH3)3), 56.4 (C-14), 56.0 (C-17), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 40.7 (C-9, CH2N), 40.3 (CH2N), 40.1 (C-12), 37.2 (C-2), 37.0 (C-1), 35.5 (C-8, C-20), 34.9 (C-10), 33.5 (C-23), 31.7 (C-22), 28.4 (CH3), 28.2 (C-16), 26.6 (C-6), 25.8 (C-7), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C31H52N2O4: C, 72.05; H, 10.14; found C, 72.00; H, 10.09. MALDI TOF: m/z 539.387 ([M + Na]+, calcd 539.382), 555.362 ([M + K]+, calcd 555.356).
tert-Butyl Butyl (4-{[(5β)-3,24-Dioxocholan-24-yl)amino]butyl}carbamate (20b)
White waxy solid, 0.55 g, 85% yield. [α]D16 + 19.3 (c 0.97, CHCl3); IR (KBr) νmax 2929, 2865, 1711, 1650, 1536, 1452, 1379, 1269, 1252, 1173, 756, 666 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.11 (1H, br s, NH), 4.71 (1H, br s, NH), 3.27–3.23 (2H, m, CH2N), 3.14–3.10 (2H, m, CH2N), 2.71‒1.05 (28H, m), 1.49–1.44 (4H, m, CH2), 1.43 (9H, s, CH3), 1.01 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.67 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.4 (C-3), 173.9 (C-24), 156.2 (COO), 79.2 (C(CH3)3), 56.4 (C-14), 56.0 (C-17), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 40.7 (C-9, CH2N), 40.1 (C-12), 39.2 (CH2N), 37.2 (C-2), 37.0 (C-1), 35.5 (C-8, C-20), 34.9 (C-10), 33.5 (C-23), 31.9 (C-22), 28.4 (CH3), 28.2 (C-16), 27.7 (CH2), 26.6 (C-6, CH2), 25.8 (C-7), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C33H56N2O4: C, 72.75; H, 10.36; found C, 72.69; H, 10.33. MALDI TOF: m/z 689.415 ([M + Na]+, calcd 689.512), 705.377 ([M + K]+, calcd 705.486).
tert-Butyl (6-{[(5β)-3,24-Dioxocholan-24-yl)amino]hexyl}carbamate (20c)
Colorless waxy solid, 0.58 g, 85% yield. [α]D14 + 14.0 (c 0.95, CHCl3); IR (KBr) νmax 2932, 2864, 1710, 1649, 1539, 1454, 1378, 1271, 1251, 1173, 754, 666 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.95 (1H, br s, NH), 4.68 (1H, br s, NH), 3.20–3.16 (2H, m, CH2N), 3.08–3.04 (2H, m, CH2N), 2.68‒1.02 (28H, m), 1.47–1.40 (4H, m, CH2), 1.39 (9H, s, CH3), 1.30–1.26 (4H, m, CH2), 0.97 (3H, s, H-19), 0.89 (3H, d, J = 6.5 Hz, H-21), 0.64 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.4 (C-3), 173.6 (C-24), 156.1 (COO), 78.9 (C(CH3)3), 56.4 (C-14), 56.0 (C-17), 44.3 (C-5), 42.7 (C-13), 42.3 (C-4), 40.7 (C-9), 40.2 (CH2N), 40.0 (C-12), 39.1 (CH2N), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8, C-20), 34.8 (C-10), 33.6 (C-23), 31.8 (C-22), 29.9 (CH2), 29.5 (CH2), 28.4 (CH3), 28.2 (C-16), 26.6 (C-6), 26.2 (CH2), 26.1 (CH2), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C35H60N2O4: C, 73.38; H, 10.56; found C, 73.29; H, 10.53. MALDI TOF: m/z 595.475 ([M + Na]+, calcd 595.445), 611.439 ([M + K]+, calcd 611.419).
tert-Butyl 4-(3,24-Dioxocholan-24-yl)piperazine-1-carboxylate (20d)
Colorless waxy solid, 0.53 g, 83% yield. [α]D21 + 16.6 (c 0.86, CHCl3); IR (KBr) νmax 2932, 2865, 1699, 1647, 1455, 1419, 1365, 1285, 1253, 1168, 1129, 1026, 997, 754, 665 cm−1; 1H NMR (CDCl3, 500 MHz) δ 3.60–3.55 (2H, m, CH2N), 3.48–3.40 (6H, m, CH2N), 2.73‒1.06 (28H, m), 1.48 (9H, s, CH3), 1.02 (3H, s, H-19), 0.95 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.4 (C-3), 172.3 (C-24), 154.6 (COO), 80.3 (C(CH3)3), 56.4 (C-14), 56.1 (C-17), 45.5 (CH2N), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 41.3 (CH2N), 40.7 (C-9), 40.1 (C-12), 37.2 (C-2), 37.0 (C-1), 35.6 (C-8), 35.5 (C-20), 34.9 (C-10), 31.4 (C-22), 30.4 (C-23), 28.4 (CH3), 28.3 (C-16), 26.6 (C-6), 25.8 (C-7), 24.2 (C-15), 22.7 (C-19), 21.2 (C-11), 18.5 (C-21), 12.1 (C-18); anal. calcd for C33H54N2O4: C, 73.02; H, 10.03; found C, 72.93; H, 9.99. MALDI TOF: m/z 565.379 ([M + Na]+, calcd 565.398), 581.346 ([M + K]+, calcd 581.372).
3.9.8. General Procedure for Removing BOC-Protected Groups
Trifluoroacetic acid (3.4 mL) was added to a solution of 19a–d or 20a–d (1.0 mmol) in CHCl3 (20 mL), and the mixture was stirred at room temperature for 1 h. The solvent was then evaporated under reduced pressure, and the residue was purified by chromatography on silica gel using CHCl3/MeOH (10:1) as the mobile phase, affording the product 21a–d or 22a–d.
(3α,5β)-24-[(2-Aminoethyl)amino]-24-oxocholan-3-yl Acetate (21a)
White crystals, 0.45 g, 98% yield. MP 34–36 °С, [α]D19 + 23.9 (c 0.69, CHCl3); IR (KBr) νmax 2931, 2868, 1681, 1541, 1449, 1383, 1364, 1247, 1203, 1181, 1138, 1028, 981, 837, 799, 756, 666, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.11 (2H, br s, NH2), 7.55 (1H, br s, NH), 4.73–7.68 (1H, m, H-3), 3.56–3.46 (2H, m, CH2NH), 3.15–3.06 (2H, m, CH2NH2), 2.27‒1.02 (28H, m), 2.01 (3H, s, CH3CO), 0.92 (3H, s, H-19), 0.91 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 176.6 (C-24), 170.7 (CH3CO), 74.4 (C-3), 56.4 (C-14), 56.1 (C-17), 42.7 (C-13), 41.8 (C-5), 40.4 (C-9), 40.1 (C-12), 40.0 (CH2NH2), 37.3 (CH2NH), 35.8 (C-8), 35.6 (C-20), 35.0 (C-1), 34.6 (C-10), 33.2 (C-23), 32.2 (C-4), 31.6 (C-22), 28.2 (C-16), 27.0 (C-6), 26.6 (C-2), 26.3 (C-7), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.2 (C-21), 11.9 (C-18); anal. calcd for C28H48N2O3: C, 73.00; H, 10.50; found C, 72.95; H, 10.46.
(3α,5β)-24-[(4-Aminobutyl)amino]-24-oxocholan-3-yl Acetate (21b)
White solid, 0.48 g, 98% yield. [α]D17 + 18.0 (c 0.89, CHCl3); IR (KBr) νmax 2937, 2868, 1678, 1647, 1550, 1448, 1381, 1363, 1245, 1203, 1179, 1135, 1028, 836, 799, 755, 722, 665 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.97 (2H, br s, NH2), 6.77 (1H, br s, NH), 4.73–4.68 (1H, m, H-3), 3.23–3.17 (2H, m, CH2NH), 2.99–2.93 (2H, m, CH2NH2), 2.25‒1.00 (28H, m), 2.02 (3H, s, CH3CO), 1.72–1.66 (2H, m, CH2), 1.58–1.52 (2H, m, CH2), 0.93 (3H, s, H-19), 0.90 (3H, d, J = 6.5 Hz, H-21), 0.64 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.9 (C-24), 170.6 (CH3CO), 74.4 (C-3), 56.5 (C-14), 56.1 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.2 (C-12), 39.4 (CH2NH2), 38.6 (CH2NH), 35.8 (C-8), 35.6 (C-20), 35.0 (C-1), 34.6 (C-10), 33.4 (C-23), 32.3 (C-4), 31.6 (C-22), 28.2 (C-16), 27.0 (C-6), 26.6 (C-2), 26.4 (C-7), 26.2 (CH2), 24.4 (CH2), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C30H52N2O3: C, 73.72; H, 10.72; found C, 73.67; H, 10.69.
(3α,5β)-24-[(6-Aminohexyl)amino]-24-oxocholan-3-yl Acetate (21c)
White solid, 0.50 g, 97% yield. [α]D24 + 17.3 (c 0.96, CHCl3); IR (KBr) νmax 2937, 2866, 1680, 1644, 1548, 1448, 1380, 1363, 1244, 1202, 1179, 1137, 1041, 979, 933, 909, 835, 799, 755, 722, 667, 549 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.97 (2H, br s, NH2), 6.41 (1H, br s, NH), 4.73–4.68 (1H, m, H-3), 3.18–3.15 (2H, m, CH2NH), 2.95–2.90 (2H, m, CH2NH2), 2.26‒0.96 (28H, m), 2.02 (3H, s, CH3CO), 1.70–1.65 (2H, m, CH2), 1.50–1.45 (2H, m, CH2), 1.36–1.29 (4H, m, CH2), 0.93 (3H, s, H-19), 0.91 (3H, d, J = 6.5 Hz, H-21), 0.64 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 174.5 (C-24), 170.6 (CH3CO), 74.4 (C-3), 56.5 (C-14), 56.1 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.2 (C-12), 39.7 (CH2NH2), 39.2 (CH2NH), 35.8 (C-8), 35.6 (C-20), 35.0 (C-1), 34.6 (C-10), 33.5 (C-23), 32.2 (C-4), 31.9 (C-22), 28.9 (CH2), 28.2 (C-16), 27.0 (C-6, CH2), 26.6 (C-2), 26.3 (C-7), 25.9 (CH2), 25.6 (CH2), 24.2 (C-15), 23.3 (C-19), 21.5 (CH3CO), 20.8 (C-11), 18.3 (C-21), 12.0 (C-18); anal. calcd for C32H56N2O3: C, 74.37; H, 10.92; found C, 74.32; H, 10.89.
(3α,5β)-24-Oxo-24-piperazin-1-ylcholan-3-yl Acetate (21d)
White solid; 0.47 g, 97% yield. [α]D24 + 20.5 (c 0.99, CHCl3); IR (KBr) νmax 2938, 2867, 1727, 1674, 1447 1431, 1383, 1363, 1246, 1202, 1180, 1134, 1028, 833, 799, 755, 722, 666, 564 cm−1; 1H NMR (CDCl3, 500 MHz) δ 10.02 (1H, br s, NH), 4.71–4.67 (1H, m, H-3), 3.88–3.77 (4H, m, CH2N), 3.22–3.17 (4H, m, CH2NH), 2.38‒0.98 (28H, m), 2.00 (3H, s, CH3CO), 0.91 (3H, s, H-19), 0.91 (3H, d, J = 6.5 Hz, H-21), 0.63 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 172.3 (C-24), 170.6 (CH3CO), 74.4 (C-3), 56.4 (C-14), 55.9 (C-17), 43.4 (CH2NH), 42.7 (C-13), 42.3 (CH2N), 41.8 (C-5), 40.4 (C-9), 40.1 (C-12), 38.2 (CH2N), 35.8 (C-8), 35.5 (C-20), 35.0 (C-1), 34.5 (C-10), 32.2 (C-4), 31.1 (C-22), 29.9 (C-23), 28.3 (C-16), 26.9 (C-6), 26.6 (C-2), 26.3 (C-7), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); anal. calcd for C30H50N2O3: C, 74.03; H, 10.35; found C, 73.96; H, 10.32.
(5β)-N-(2-Aminoethyl)-3-oxocholan-24-amide (22a)
Colorless waxy solid, 0.41 g, 98% yield. [α]D19 + 17.0 (c 0.88, CHCl3); IR (KBr) νmax 2927, 2866, 1678, 1547, 1454, 1379, 1339, 1256, 1202, 1181, 1136, 836, 799, 755 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.27 (2H, br s, NH2), 7.67 (1H, br s, NH), 3.54–3.48 (2H, m, CH2NH), 3.15–3.10 (2H, m, CH2NH2), 2.71‒1.04 (28H, m), 1.00 (3H, s, H-19), 0.90 (3H, d, J = 6.5 Hz, H-21), 0.67 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 212.1 (C-3), 175.7 (C-24), 56.3 (C-14), 56.0 (C-17), 44.3 (C-5), 42.7 (C-13), 42.2 (C-4), 40.6 (C-9), 40.0 (C-12, CH2NH2), 37.2 (C-2), 36.9 (C-1, CH2NH), 35.5 (C-8, C-20), 34.8 (C-10), 32.9 (C-23), 31.5 (C-22), 28.1 (C-16), 26.6 (C-6), 25.7 (C-7), 24.1 (C-15), 22.4 (C-19), 21.1 (C-11), 18.1 (C-21), 11.8 (C-18); anal. calcd for C26H44N2O2: C, 74.95; H, 10.64; found C, 74.89; H, 10.60.
(5β)-N-(4-Aminobutyl)-3-oxocholan-24-amide (22b)
Colorless waxy solid, 0.43 g, 97% yield. [α]D14 + 11.0 (c 0.98, CHCl3); IR (KBr) νmax 2936, 2867, 1682, 1646, 1557, 1542, 1456, 1379, 1339, 1268, 1202, 1179, 1134, 835, 799, 753, 722 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.14 (2H, br s, NH2), 6.79 (1H, br s, NH), 3.23–3.20 (2H, m, CH2NH), 2.99–2.95 (2H, m, CH2NH2), 2.73‒1.05 (28H, m), 1.73–1.69 (2H, m, CH2), 1.59–1.55 (2H, m, CH2), 1.03 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.68 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 174.9 (C-24), 56.4 (C-14), 55.9 (C-17), 44.2 (C-5), 42.8 (C-13), 42.3 (C-4), 40.7 (C-9), 40.0 (C-12), 39.4 (CH2NH2), 38.5 (CH2NH), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8, C-20), 34.9 (C-10), 33.2 (C-23), 31.8 (C-22), 28.2 (C-16), 26.6 (C-6), 26.2 (CH2), 25.8 (C-7), 24.5 (CH2), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C28H48N2O2: C, 75.63; H, 10.88; found C, 75.57; H, 10.83. MALDI TOF: m/z 445.377 ([M + H]+, calcd 445.379).
(5β)-N-(6-Aminohexyl)-3-oxocholan-24-amide (22c)
Colorless waxy solid, 0.45 g, 97% yield. [α]D21 + 15.5 (c 0.82, CHCl3); IR (KBr) νmax 2935, 2865, 1682, 1638, 1557, 1542, 1456, 1380, 1268, 1202, 1180, 1135, 835, 799, 755, 722 cm−1; 1H NMR (CDCl3, 500 MHz) δ 8.07 (2H, br s, NH2), 6.42 (1H, br s, NH), 3.19–3.16 (2H, m, CH2NH), 2.95–2.91 (2H, m, CH2NH2), 2.70‒1.05 (28H, m), 1.69–1.65 (2H, m, CH2), 1.49–1.46 (2H, m, CH2), 1.37–1.31 (4H, m, CH2), 1.03 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 174.4 (C-24), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.8 (C-13), 42.3 (C-4), 40.7 (C-9), 40.1 (C-12), 39.6 (CH2NH2), 39.1 (CH2NH), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8, C-20), 34.9 (C-10), 33.4 (C-23), 31.9 (C-22), 29.0 (CH2), 28.2 (C-16), 27.1 (CH2), 26.6 (C-6), 25.9 (CH2), 25.8 (C-7), 25.6 (CH2), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.3 (C-21), 12.1 (C-18); anal. calcd for C30H52N2O2: C, 76.22; H, 11.09; found C, 76.17; H, 11.05. MALDI TOF: m/z 511.362 ([M + K]+, calcd 511.367).
(5β)-24-Oxo-24-piperazin-1-ylcholan-3-one (22d)
Colorless waxy solid, 0.42 g, 96% yield. [α]D17 +10.0 (c 0.89, CHCl3); IR (KBr) νmax 2934, 2866, 2498, 1681, 1443 1378, 1203, 1181, 1135, 1026, 835, 800, 753, 721, 667 cm−1; 1H NMR (CDCl3, 500 MHz) δ 9.59 (1H, br s, NH), 3.91–3.77 (4H, m, CH2N), 3.28–3.22 (4H, m, CH2NH), 2.71‒1.04 (28H, m), 1.02 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.68 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.8 (C-3), 172.8 (C-24), 56.4 (C-14), 55.8 (C-17), 44.2 (C-5), 43.5 (CH2NH), 42.8 (C-13), 42.3 (C-4), 42.3, 38.4 (CH2N), 40.7 (C-9), 40.0 (C-12), 37.2 (C-2), 36.9 (C-1), 35.5 (C-8, C-20), 34.8 (C-10), 31.0 (C-22), 29.8 (C-23), 28.2 (C-16), 26.6 (C-6), 25.7 (C-7), 24.1 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.0 (C-18); anal. calcd for C28H46N2O2: C, 75.97; H, 10.47; found C, 75.95; H, 10.46. MALDI TOF: m/z 465.341 ([M + Na]+, calcd 465.346), 481.301 ([M + Na]+, calcd 481.319).
3.9.9. Reaction of Diaminoalkane Derivatives of 3a-Acetoxy-5β-cholan-24-oic Acid and 3-Oxo-cholan-24-oic Acid 21a–d, 22a–d with (5Z,9Z)-Tetradeca-5,9-dienedioic Acid (5)
Lithocholic acid derivative
23a–
d and
24a–
d was synthesized according to a modified literature procedure [
41]. To a solution of diaminoalkane derivatives of 3α-acetoxy-5β-cholan-24-oic acid
21a–
d or 3-oxo-cholan-24-oic acid
22a–
d (1.0 mmol) in CH
2Cl
2 (50 mL), (5
Z,9
Z)-tetradeca-5,9-dienedioic acid (
5) (0.51 g, 2.0 mmol) was added, followed by EDC·HCl (0.48 g, 2.5 mmol) and DMAP (18 mg, 0.15 mmol) under argon. The mixture was stirred at room temperature for 12 h until the reaction was complete (TLC monitoring, hexane/ethyl acetate). The mixture was diluted with H
2O (10 mL) and the CH
2Cl
2 layer was separated, dried over MgSO
4, and concentrated. The crude product was purified by column chromatography (silica gel) using CHCl
3/MeOH (10:1) as the elution solvent to afford compounds
23a–
d or
24a–
d.
(5Z,9Z)-14-[(2-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]amino}ethyl)amino]-14-oxotetradeca-5,9-dienoic Acid (23a)
Colorless waxy solid, 0.47 g, 68% yield. [α]D19 + 16.1 (c 0.76, CHCl3); IR (KBr) νmax 2929, 2866, 1735, 1647, 1546, 1449, 1379, 1363, 1243, 1065, 1028, 756, 666, 608 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.08 (1H, br s, NH), 6.85 (1H, br s, NH), 5.43–5.33 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.73–4.68 (1H, m, H-3), 3.39–3.33 (4H, m, CH2NH), 2.37‒1.00 (28H, m), 2.37–2.34 (2H, m, H-2’), 2.23–2.19 (2H, m, H-13’), 2.10–2.04 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.03 (3H, s, CH3CO), 1.73–1.66 (4H, m, H-3’, H-12’), 0.93 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.64 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 176.2 (C-1’), 175.1 (C-24), 174.5 (C-14’), 170.7 (CH3CO), 130.2, 130.1 (C-6’, C-9’), 129.1 (C-5’, C-10’), 74.4 (C-3), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 39.9, 39.7 (CH2NH), 35.9 (C-13’), 35.8 (C-8), 35.5 (C-20), 35.0 (C-1), 34.6 (C-10), 33.5 (C-23), 33.1 (C-2’), 32.2 (C-4), 31.8 (C-22), 28.2 (C-16), 27.7, 27.5 (C-7’, C-8’), 27.3 (C-11’), 27.0 (C-6), 26.7 (C-4’), 26.6 (C-2), 26.3 (C-7), 25.6 (C-12’), 24.7 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C42H68N2O6: C, 72.37; H, 9.83; found C, 72.30; H, 9.79; MALDI TOF: m/z 719.428 ([M + Na]+, calcd 719.498), 735.508 ([M + K]+, calcd 735.471).
(5Z,9Z)-14-[(4-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]amino}butyl)amino]-14-oxotetradeca-5,9-dienoic Acid (23b)
Colorless waxy solid, 0.49 g, 68% yield. [α]D19 + 13.5 (c 0.37, CHCl3); IR (KBr) νmax 2932, 2864, 1734, 1647, 1541, 1456, 1383, 1363, 1243, 1165, 1029, 800, 753 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.49 (1H, br s, NH), 6.11 (1H, br s, NH), 5.47–5.35 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.73–4.68 (1H, m, H-3), 3.30–3.25 (4H, m, CH2NH), 2.36 (2H, t, J = 7.2 Hz, H-2’), 2.30‒1.00 (28H, m), 2.24 (2H, t, J = 7.2 Hz, H-13’), 2.11–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.04 (3H, s, CH3CO), 1.74–1.66 (4H, m, H-3’, H-12’), 1.57–1.51 (4H, m, CH2), 0.94 (3H, s, H-19), 0.92 (3H, d, J = 6.8 Hz, H-21), 0.65 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 176.1 (C-1’), 174.6 (C-24), 174.3 (C-14’), 170.7 (CH3CO), 130.3, 130.1 (C-6’, C-9’), 129.2 (C-5’, C-10’), 74.4 (C-3), 56.5 (C-14), 56.1 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.2 (C-12), 39.9 (С-25, С-28), 36.1 (C-13’), 35.8 (C-8), 35.5 (C-20), 35.0 (C-1), 34.6 (C-10), 33.6 (C-23), 33.3 (C-2’), 32.3 (C-4), 31.9 (C-22), 28.2 (C-16), 27.6, 27.5 (C-7’, C-8’), 27.0 (C-6), 26.9, 26.8 (C-4’, C-11’), C-26, C-27), 26.6 (C-2), 26.3 (C-7), 25.9 (C-12’), 24.8 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C44H72N2O6: C, 72.89; H, 10.01; found C, 72.88; H, 9.98; MALDI TOF: m/z 747.440 ([M + Na]+, calcd 747.529), 763.422 ([M + K]+, calcd 763.503).
(5Z,9Z)-14-[(4-{[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]amino}hexyl)amino]-14-oxotetradeca-5,9-dienoic Acid (23c)
Colorless waxy solid, 0.48 g, 65% yield. [α]D19 + 15.3 (c 0.95, CHCl3); IR (KBr) νmax 2933, 2864, 1734, 1646, 1549, 1455, 1379, 1363, 1243, 1163, 1111, 1046, 979, 933, 909, 729, 668, 549 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.36 (1H, br s, NH), 6.12 (1H, br s, NH), 5.40–5.32 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.72–4.68 (1H, m, H-3), 3.20 (4H, q, J = 6.5 Hz, CH2NH), 2.31 (2H, t, J = 7.5 Hz, H-2’), 2.27‒1.00 (28H, m), 2.19 (2H, J = 7.5 Hz, H-13’), 2.11–2.03 (8H, m, H-4’, H-7’, H-8’, H-11’), 2.00 (3H, s, CH3CO), 1.69–1.64 (4H, m, H-3’, H-12’), 1.49–1.45 (4H, m, CH2), 1.33–1.28 (4H, m, CH2), 0.90 (3H, s, H-19), 0.89 (3H, d, J = 6.8 Hz, H-21), 0.61 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 176.5 (C-1’), 174.3 (C-24), 173.9 (C-14’), 170.7 (CH3CO), 130.3, 130.2 (C-6’, C-9’), 129.1 (C-5’, C-10’), 74.4 (C-3), 56.5 (C-14), 56.0 (C-17), 42.7 (C-13), 41.9 (C-5), 40.4 (C-9), 40.1 (C-12), 39.1, 39.0 (CH2NH), 36.1 (C-13’), 35.8 (C-8), 35.5 (C-20), 35.0 (C-1), 34.6 (C-10), 33.6 (C-23), 33.5 (C-2’), 32.2 (C-4), 31.9 (C-22), 29.3, 29.2 (CH2), 28.2 (C-16), 27.5, 27.4 (C-7’, C-8’), 26.9 (C-6), 26.8 (C-11’), 26.6 (C-2), 26.4 (C-4’), 26.3 (C-7), 25.9 (C-12’, CH2), 24.8 (C-3’), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); anal. calcd for C46H76N2O6: C, 73.36; H, 10.17; found C, 73.30; H, 10.12; MALDI TOF: m/z 775.579 ([M + Na]+, calcd 775.560), 791.556 ([M + K]+, calcd 791.534).
(5Z,9Z)-14-{4-[(3α,5β)-3-(Acetyloxy)-24-oxocholan-24-yl]piperazin-1-yl}-14-oxotetradeca-5,9-dienoic Acid (23d)
Colorless waxy solid, 0.46 g, 64% yield. [α]D19 + 18.0 (c 0.94, CHCl3); IR (KBr) νmax 2935, 2866, 1732, 1647, 1432, 1379, 1363, 1243, 1179, 1026, 983, 888, 753, 666, 615 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.40–5.30 (4H, m, H-5’, H-6’, H-9’, H-10’), 4.67 (1H, m, H-3), 3.62–3.56 (4H, m, CH2N), 3.47–3.43 (4H, m, CH2N), 2.33–2.28 (4H, m, H-2’, H-13’), 2.38‒0.96 (28H, m), 2.10–2.01 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.99 (3H, s, CH3CO), 1.68–1.64 (4H, m, H-3’, H-12’), 0.90 (3H, d, J = 6.8 Hz, H-21), 0.89 (3H, s, H-19), 0.61 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 176.8 (C-1’), 172.8 (C-24), 172.4 (C-14’), 170.7 (CH3CO), 130.3, 130.1 (C-6’, C-9’), 129.1, 128.9 (C-5’, C-10’), 74.4 (C-3), 56.4 (C-14), 55.9 (C-17), 45.6, 45.4 (CH2N), 42.7 (C-13), 41.8 (C-5), 41.6, 41.3 (CH2N), 40.4 (C-9), 40.1 (C-12), 35.7 (C-8), 35.6 (C-20), 34.9 (C-1), 34.5 (C-10), 33.3 (C-13’), 32.6 (C-2’), 32.3 (C-4), 31.3 (C-22), 30.2 (C-23), 28.3 (C-16), 27.4, 27.3 (C-7’, C-8’), 26.9 (C-6), 26.7 (C-4’), 26.6 (C-2), 26.4 (C-11’), 26.3 (C-7), 25.2 (C-3’), 24.7 (C-12’), 24.2 (C-15), 23.3 (C-19), 21.4 (CH3CO), 20.8 (C-11), 18.5 (C-21), 12.0 (C-18); anal. calcd for C44H70N2O6: C, 69.34; H, 9.26; found C, 69.29; H, 9.20; MALDI TOF: m/z 745.544 ([M + Na]+, calcd 745.513), 761.515 ([M + K]+, calcd 761.487).
(5Z,9Z)-14-[(2-{[(5β)-3,24-Dioxocholan-24-yl]amino}ethyl)amino]-14-oxotetradeca-5,9-dienoic Acid (24a)
Colorless waxy solid, 0.44 g, 67% yield. [α]D16 + 11.9 (c 0.91, CHCl3); IR (KBr) νmax 2929, 2865, 1713, 1651, 1550, 1455, 1379, 1257, 1165, 1150, 1076, 1023, 754, 665 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.04 (1H, br s, NH), 6.85 (1H, br s, NH), 5.44–5.30 (4H, m, H-5’, H-6’, H-9’, H-10’), 3.38–3.34 (4H, m, CH2NH), 2.70‒1.05 (28H, m), 2.38–2.34 (2H, m, H-2’), 2.22–2.18 (2H, m, H-13’), 2.10–2.04 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.70–1.64 (4H, m, H-3’, H-12’), 1.01 (3H, s, H-19), 0.91 (3H, d, J = 6.5 Hz, H-21), 0.67 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 176.3 (C-1’), 175.6 (C-24), 175.0 (C-14’), 130.2, 130.1 (C-6’, C-9’), 129.2, 129.0 (C-5’, C-10’), 56.4 (C-14), 55.9 (C-17), 44.3 (C-5), 42.7 (C-13), 42.4 (C-4), 40.7 (C-9), 40.0 (C-12), 39.7, 39.6 (CH2NH), 37.2 (C-2), 36.9(C-1), 35.9 (C-13’), 35.5 (C-8, C-20), 34.9 (C-10), 33.4 (C-23), 33.2 (C-2’), 31.8 (C-22), 28.2 (C-16), 27.6, 27.5 (C-7’, C-8’), 26.7 (C-11’), 26.6 (C-6), 26.4 (C-4’), 25.8 (C-7), 25.7 (C-12’), 24.7 (C-3’), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C40H64N2O5: C, 73.58; H, 9.88; found C, 73.54; H, 9.84; MALDI TOF: m/z 675.307 ([M + Na]+, calcd 675.482), 691.276 ([M + K]+, calcd 691.482).
(5Z,9Z)-14-[(4-{[(5β)-3,24-Dioxocholan-24-yl]amino}butyl)amino]-14-oxotetradeca-5,9-dienoic Acid (24b)
Colorless waxy solid, 0.45 g, 66% yield. [α]D17 + 15.0 (c 0.77, CHCl3); IR (KBr) νmax 2932, 2865, 1712, 1645, 1551, 1446, 1379, 1230, 1181, 1149, 1077, 1022, 754, 666 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.54 (1H, br s, NH), 6.25 (1H, br s, NH), 5.42–5.33 (4H, m, H-5’, H-6’, H-9’, H-10’), 3.28–3.24 (4H, m, CH2NH), 2.72‒1.05 (28H, m), 2.37–2.33 (2H, m, H-2’), 2.22–2.18 (2H, m, H-13’), 2.11–2.04 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.71–1.66 (4H, m, H-3’, H-12’), 1.55–1.51 (4H, m, CH2), 1.02 (3H, s, H-19), 0.93 (3H, d, J = 6.5 Hz, H-21), 0.68 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 176.2 (C-1’), 174.5 (C-24), 174.2 (C-14’), 130.3, 130.1 (C-6’, C-9’), 129.2 (C-5’, C-10’), 56.4 (C-14), 56.0 (C-17), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 40.7 (C-9), 40.1 (C-12), 39.1, 39.0 (CH2NH), 37.2 (C-2), 36.9 (C-1), 36.1 (C-13’), 35.5 (C-8, C-20), 34.9 (C-10), 33.6 (C-23), 33.4 (C-2’), 31.9 (C-22), 28.2 (C-16), 27.6, 27.5 (C-7’, C-8’), 26.9, 26.8 (CH2), 26.7 (C-11’), 26.6 (C-6), 26.4 (C-4’), 25.9 (C-12’), 25.8 (C-7), 24.7 (C-3’), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C42H68N2O5: C, 74.07; H, 10.06; found C, 74.01; H, 10.02; MALDI TOF: m/z 681.472 ([M + H]+, calcd 681.521), 703.444 ([M + Na]+, calcd 703.503), 719.407 ([M + K]+, calcd 719.477).
(5Z,9Z)-14-[(6-{[(5β)-3,24-Dioxocholan-24-yl]amino}hexyl)amino]-14-oxotetradeca-5,9-dienoic Acid (24c)
Colorless waxy solid, 0.45 g, 64% yield. [α]D23 + 11.0 (c 0.75, CHCl3); IR (KBr) νmax 2929, 2861, 1713, 1645, 1551, 1454, 1378, 1260, 1181, 1149, 1103, 1077, 1022, 991, 801, 755 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.16 (1H, br s, NH), 5.88 (1H, br s, NH), 5.47–5.34 (4H, m, H-5’,H-6’, H-9’, H-10’), 3.28–3.24 (4H, m, CH2NH), 2.73‒1.05 (28H, m), 2.38–2.33 (2H, m, H-2’), 2.25–2.21 (2H, m, H-13’), 2.12–2.05 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.74–1.67 (4H, m, H-3’, H-12’), 1.53–1.48 (4H, m, CH2), 1.38–1.33 (4H, m, CH2), 1.03 (3H, s, H-19), 0.94 (3H, d, J = 6.5 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 176.5 (C-1’), 174.2 (C-14’, C-24), 130.2, 130.1 (C-6’, C-9’), 129.1 (C-5’, C-10’), 56.4 (C-14), 56.0 (C-17), 44.3 (C-5), 42.8 (C-13), 42.4 (C-4), 40.7 (C-9), 40.1 (C-12), 39.1, 38.9 (CH2NH), 37.2 (C-2), 37.0 (C-1), 36.2 (C-13’), 35.5 (C-8, C-20), 34.9 (C-10), 33.6 (C-23), 33.4 (C-2’), 31.9 (C-22), 29.3, 29.1 (CH2), 28.2 (C-16), 27.6, 27.5 (C-7’, C-8’), 26.8 (C-11’), 26.6 (C-6), 26.4 (C-4’), 26.0, 25.8, (C-7, C-12’, CH2), 24.8 (C-3’), 24.2 (C-15), 22.7 (C-19), 21.2 (C-11), 18.4 (C-21), 12.1 (C-18); anal. calcd for C44H72N2O5: C, 74.53; H, 10.24; found C, 74.48; H, 10.19; MALDI TOF: m/z 709.589 ([M + H]+, calcd 709.552), 731.571 ([M + Na]+, calcd 731.534), 747.541 ([M + K]+, calcd 747.508).
(5Z,9Z)-{4-[(5β)-3,24-Dioxocholan-24-yl]piperazin-1-yl}-14-oxotetradeca-5,9-dienoic Acid (24d)
Colorless waxy solid, 0.43 g, 63% yield. [α]D18 + 13.1 (c 0.95, CHCl3); IR (KBr) νmax 2931, 2864, 1715, 1647, 1434, 1363, 1245, 1221, 1180, 1077, 1019, 802, 753 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.44–5.31 (4H, m, H-5’, H-6’, H-9’, H-10’), 3.66–3.60 (4H, m, CH2N), 3.52–3.45 (4H, m, CH2N), 2.40‒0.96 (28H, m), 2.39–2.33 (4H, m, H-2’, H-13’), 2.14–2.03 (8H, m, H-4’, H-7’, H-8’, H-11’), 1.72–1.66 (4H, m, H-3’, H-12’), 1.02 (3H, s, H-19), 0.95 (3H, d, J = 6.8 Hz, H-21), 0.69 (3H, s, H-18); 13C NMR (CDCl3, 125 MHz) δ 213.6 (C-3), 176.7 (C-1’), 172.6 (C-14’, C-24), 130.4, 130.2 (C-6’, C-9’), 129.1, 128.9 (C-5’, C-10’), 56.4 (C-14), 55.9 (C-17), 45.6, 45.4 (CH2N), 44.3 (C-5), 42.8 (C-13), 42.3 (C-4), 41.7, 41.6 (CH2N), 40.4 (C-9), 40.0 (C-12), 37.2 (C-2), 36.9 (C-1), 35.6 (C-8), 35.5 (C-20), 34.9 (C-10), 33.2 (C-2’), 32.6 (C-13’), 31.3 (C-22), 30.3 (C-23), 28.3 (C-16), 27.5, 27.4 (C-7’, C-8’), 26.8 (C-4’), 26.6 (C-6), 26.4 (C-11’), 25.8 (C-7), 25.3 (C-3’), 24.7 (C-12’), 24.2 (C-15), 22.6 (C-19), 21.2 (C-11), 18.5 (C-21), 12.1 (C-18); anal. calcd for C42H66N2O5: C, 74.29; H, 9.80; found C, 74.23; H, 9.77; MALDI TOF: m/z 679.503 ([M + H]+, calcd 679.497), 701.477 ([M + Na]+, calcd 701.497), 717.443 ([M + K]+, calcd 717.497).