Possible Antidepressant Effects of Memantine—Systematic Review with a Case Study
Abstract
:1. Introduction
2. Results
2.1. Preclinical Studies of Antidepressant Effect of Memantine
2.2. Clinical Studies on Antidepressant Effect of Memantine
2.3. Individual Case Report
3. Discussion
4. Methods
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Post, R.M. Treatment of Bipolar Depression. Psychiatr. Clin. N. Am. 2016, 39, 11–33. [Google Scholar] [CrossRef]
- Szmulewicz, A.G.; Angriman, F.; Samamé, C.; Ferraris, A.; Vigo, D.; Strejilevich, S.A. Dopaminergic agents in the treatment of bipolar depression: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2017, 135, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Blanpied, T.A.; Clarke, R.J.; Johnson, J.W. Amantadine Inhibits NMDA Receptors by Accelerating Channel Closure during Channel Block. J. Neurosci. 2005, 25, 3312–3322. [Google Scholar] [CrossRef] [Green Version]
- Lipton, S.A. Pathologically-Activated Therapeutics for Neuroprotection: Mechanism of NMDA Receptor Block by Memantine and S-Nitrosylation. Curr. Drug Targets 2007, 8, 621–632. [Google Scholar] [CrossRef]
- Song, X.; Jensen, M.Ø.; Jogini, V.; Stein, R.A.; Lee, C.-H.; Mchaourab, H.S.; Shaw, D.E.; Gouaux, E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nat. Cell Biol. 2018, 556, 515–519. [Google Scholar] [CrossRef]
- Johnson, J.W.; Kotermanski, S.E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 2006, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Ishizuka, T.; Yabuki, Y.; Shioda, N.; Sasaki, Y.; Tagashira, H.; Yawo, H.; Yeh, J.Z.; Sakagami, H.; Narahashi, T.; et al. Blockade of the KATP channel Kir6.2 by memantine represents a novel mechanism relevant to Alzheimer’s disease therapy. Mol. Psychiatry 2018, 23, 211–221. [Google Scholar] [CrossRef]
- Motawaj, M.; Burban, A.; Davenas, E.; Arrang, J.-M. Activation of Brain Histaminergic Neurotransmission: A Mechanism for Cognitive Effects of Memantine in Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2010, 336, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-W.; Lin, T.-Y.; Wang, S.-J. Memantine depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebral cortex nerve terminals: An NMDA receptor-independent mechanism. Neurochem. Int. 2010, 57, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kloc, R.; Luchowska, E.; Wielosz, M.; Owe-Larsson, B.; Urbańska, E.M. Memantine increases brain production of kynurenic acid via protein kinase A-dependent mechanism. Neurosci. Lett. 2008, 435, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, J.; Bao, X.; Cai, Y.; Zhao, J.; Huang, J.; Huang, W.; Fan, X.; Xu, H. Protection of Radial Glial-Like Cells in the Hippocampus of APP/PS1 Mice: A Novel Mechanism of Memantine in the Treatment of Alzheimer’s Disease. Mol. Neurobiol. 2015, 52, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Matsunaga, S.; Iwata, N. A Meta-Analysis of Memantine for Depression. J. Alzheimer’s Dis. 2017, 57, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Gunn, A.D.; Barkay, G.; Karne, H.S.; Nurnberger, I.J.; Mathew, S.J.; Ghosh, S. Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: A double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2012, 14, 64–70. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chen, S.-L.; Chang, Y.-H.; Chu, C.-H.; Huang, S.-Y.; Tzeng, N.-S.; Wang, C.-L.; Yeh, T.L.; Lu, R.-B.; Yang, Y.K. Genotype variant associated with add-on memantine in bipolar II disorder. Int. J. Neuropsychopharmacol. 2013, 17, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelecki, D.; Tabaszewska, A.; Barszcz, Z.; Józefowicz, O.; Kropiwnicki, P.; Rabe-Jabłońska, J. A 10-Week Memantine Treatment in Bipolar Depression: A Case Report. Focus on Depressive Symptomatology, Cognitive Parameters and Quality of Life. Psychiatry Investig. 2013, 10, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.; Bies, R.R.; Shekhar, A.; Anand, A. Bayesian model of Hamilton Depression Rating Score (HDRS) with memantine augmentation in bipolar depression. Br. J. Clin. Pharmacol. 2013, 75, 791–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzystanek, M.; Pałasz, A. Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression—Case Series Study. Pharmaceuticals 2020, 13, 326. [Google Scholar] [CrossRef]
- Moryl, E.; Danysz, W.; Quack, G. Potential Antidepressive Properties of Amantadine, Memantine and Bifemelane. Pharmacol. Toxicol. 1993, 72, 394–397. [Google Scholar] [CrossRef]
- Rogóz, Z.; Skuza, G.; Maj, J.; Danysz, W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002, 42, 1024–1030. [Google Scholar] [CrossRef]
- Almeida, R.C.; Souza, D.G.; Soletti, R.C.; López, M.G.; Rodrigues, A.L.S.; Gabilan, N.H. Involvement of PKA, MAPK/ERK and CaMKII, but not PKC in the acute antidepressant-like effect of memantine in mice. Neurosci. Lett. 2006, 395, 93–97. [Google Scholar] [CrossRef]
- Réus, G.Z.; Stringari, R.B.; Kirsch, T.R.; Fries, G.R.; Kapczinski, F.; Roesler, R.; Quevedo, J. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: Further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res. Bull. 2010, 81, 585–589. [Google Scholar] [CrossRef]
- Quan, M.-N.; Zhang, N.; Wang, Y.-Y.; Zhang, T.; Yang, Z. Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience 2011, 182, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Stringari, R.B.; Ribeiro, K.F.; Ferraro, A.K.; Vitto, M.F.; Cesconetto, P.; Souza, C.T.; Quevedo, J. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav. Brain Res. 2011, 221, 166–171. [Google Scholar] [CrossRef]
- Amidfar, M.; Réus, G.Z.; Quevedo, J.; Kim, Y.-K.; Arbabi, M. Effect of co-administration of memantine and sertraline on the antidepressant-like activity and brain-derived neurotrophic factor (BDNF) levels in the rat brain. Brain Res. Bull. 2017, 128, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; Kim, Y.-K.; Wiborg, O. Effectiveness of memantine on depression-like behavior, memory deficits and brain mRNA levels of BDNF and TrkB in rats subjected to repeated unpredictable stress. Pharmacol. Rep. 2018, 70, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Zhong, S.; Liao, X.; Lai, S.; Shan, Y.; Chen, J.; Zhang, L.; Lu, Q.; Shen, S.; et al. Alleviation of cognitive deficits and high copper levels by an NMDA receptor antagonist in a rat depression model. Compr. Psychiatry 2020, 102, 152200. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Quiroz, J.A.; De Jesus, G.; Denicoff, K.K.; Luckenbaugh, D.A.; Manji, H.K.; Charney, D.S. A Double-Blind, Placebo-Controlled Study of Memantine in the Treatment of Major Depression. Am. J. Psychiatry 2006, 163, 153–155. [Google Scholar] [CrossRef]
- Ferguson, J.M.; Shingleton, R.N. An Open-label, Flexible-Dose Study of Memantine in Major Depressive Disorder. Clin. Neuropharmacol. 2007, 30, 136–144. [Google Scholar] [CrossRef]
- Muhonen, L.H.; Lönnqvist, J.; Juva, K.; Alho, H. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J. Clin. Psychiatry 2008, 69, 392–399. [Google Scholar] [CrossRef]
- Smith, E.G.; Deligiannidis, K.M.; Ulbricht, C.M.; Landolin, C.S.; Patel, J.K.; Rothschild, A.J. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: A randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2013, 74, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Omranifard, V.; Shirzadi, E.; Samandari, S.; Afshar, H.; Maracy, M.R. Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study. J. Res. Med. Sci. 2014, 19, 525–530. [Google Scholar]
- Amidfar, M.; Khiabany, M.; Kohi, A.; Salardini, E.; Arbabi, M.; Azizi, M.R.; Zarrindast, M.; Mohammadinejad, P.; Zeinoddini, A.; Akhondzadeh, S. Effect of memantine combination therapy on symptoms in patients with moderate-to-severe depressive disorder: Randomized, double-blind, placebo-controlled study. J. Clin. Pharm. Ther. 2016, 42, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Lavretsky, H.; Laird, K.T.; Krause-Sorio, B.; Heimberg, B.F.; Yeargin, J.; Grzenda, A.; Wu, P.; Thana-Udom, K.; Ercoli, L.M.; Siddarth, P. A Randomized Double-Blind Placebo-Controlled Trial of Combined Escitalopram and Memantine for Older Adults With Major Depression and Subjective Memory Complaints. Am. J. Geriatr. Psychiatry 2020, 28, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Koukopoulos, A.; Serra, G.; Koukopoulos, A.E.; Reginaldi, D.; Serra, G. The sustained mood-stabilizing effect of memantine in the management of treatment resistant bipolar disorders: Findings from a 12-month naturalistic trial. J. Affect. Disord. 2012, 136, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Keck, P.E.; Hsu, H.-A.; Papadakis, K.; Russo, J. Memantine Efficacy and Safety in Patients with Acute Mania Associated with Bipolar I Disorder. Clin. Neuropharmacol. 2009, 32, 199–204. [Google Scholar] [CrossRef]
- Serra, G.; De Chiara, L.; Manfredi, G.; Koukopoulos, A.E.; Sani, G.; Girardi, P.; Koukopoulos, A.; Serra, G. Memantine in the management of affective recurrences of bipolar disorders after the discontinuation of long-term lithium treatment: Three case histories. Ther. Adv. Psychopharmacol. 2013, 4, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evid.-Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J. Eval. Clin. Pract. 2010, 18, 12–18. [Google Scholar] [CrossRef]



| Author | Year | Behavioral Model | Animal Used | Dose of Memantine | Duration | Conclusions |
|---|---|---|---|---|---|---|
| Moryl et al. [18] | 1993 | Forced swim test | rat | 5, 10, and 20 mg/kg | 24, 5, and 1 h before the forced swim test | Memantine dose dependently decreased the duration of immobility time in rats. Moreover, memantine showed an antidepressant-like activity. |
| Rogóż et al. [19] | 2002 | Forced swimming test | rat | 5 mg/kg and 2.5 mg/kg | 24, 5, and 1 h before the forced swim test | Memantine, in combination with imipramine, fluoxetine, and venlafaxine, produced significant more enhanced antidepressant effect in rats than each of these drugs alone. |
| Almeida et al. [20] | 2006 | Forced swimming test | mice | 3–10 mg/kg | 30 min before the forced swim test | Acute antidepressant-like effect of memantine seemed to be dependent on the cellular signaling modulated by PKA, CaMKII, and MAPK/ERK, but not by PKC. |
| Réus et al. [21] | 2010 | Forced swimming test | rat | 5, 10, and 20 mg/kg | Both 2 weeks (chronic treatment) and one single time (acute treatment) | Acute and chronic administration of memantine at all doses decreased the immobility time of rats, but only acute treatment with memantine enhanced the protein levels of BDNF in the rat hippocampus. |
| Quan et al. [22] | 2011 | 21 days of exposure to chronic unpredictable stress | rat | 20 mg/kg | 3 weeks | Memantine improved the sucrose consumption, reversal learning, and prefrontal cortical synaptic plasticity, but impaired spatial memory, which is probably due to different extent of up-regulating NR2B receptor expression in prefrontal cortex and hippocampus in stressed rats. |
| Réus et al. [23] | 2011 | 40 days of exposure to chronic mild stress | rat | 20 mg/kg | 1 week | Memantine normalized anhedonia, corticosterone levels, and hypertrophic adrenal gland, and increased BDNF protein levels in the rat prefrontal cortex. |
| Amidfar et al. [24] | 2017 | Forced swimming test | rat | 2.5 and 5 mg/kg | 2 weeks | Co-administration of antidepressant memantine with sertraline induced a more pronounced antidepressant activity than treatment with each antidepressant alone. Antidepressant properties using the combination of memantine and sertraline could be attributed to increased levels of BDNF. |
| Amidfar et al. [25] | 2018 | 10 days of exposure to repeated unpredictable stress | rat | 20 mg/kg | 2 weeks | The administration of memantine reversed depression-like behavior and memory impairment, and significantly increased BDNF and TrkB mRNA levels in both the prefrontal cortex and hippocampus of stress exposed rats. |
| Li et al. [26] | 2020 | 3 weeks corticosterone or/and copper gluconate administration | rat | 20 mg/kg | 2 weeks | The results of behavioral tests and cognitive function after memantine treatment were significantly normalized, and the copper concentration was decreased in all of the groups. |
| Authors | Year | Type of Study | Sample Size | Characteristic of Participants | Intervention | Results | Conclusions | QATQS Global Rating |
|---|---|---|---|---|---|---|---|---|
| Zarate et al. [27]. | 2006 | RCT | 32 | Subjects with major depression | Memantine (5–20 mg/day) (n = 16) or placebo (n = 16) for 8 weeks. Primary efficacy was assessed by performance on the Montgomery–Asberg Depression Rating Scale (MADRS). | The linear mixed models for total MADRS scores showed no treatment effect. | In an 8-week trial, memantine in doses of 5–20 mg/day was not effective in the treatment of major depressive disorder. | 1 |
| Ferguson and Shingleton [28] | 2007 | Non RCT | 8 | Subjects with major depression | In this 12-week subjects were treated for 4 weeks to 20 mg/d memantine. Nonresponsive patients were titrated to 30 mg/d (at week 8) or 40 mg/d (at week 10). Outcome measures were MADRS, HDRS, Clinical Global Impression-Severity of Illness and the Clinical Global Impression-Improvement Scales, Patient Global Evaluation, and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Major Depressive Episode Checklist. | Baseline MADRS score was 31.9 (4.45), indicative of severe depression. Seven subjects completed the study. All patients attained the target dose of 20 mg/d; 3 patients were titrated to 30 mg/d after week 8, and 2 patients were titrated to 40 mg/d after week 10. The mean dosage across all weeks was 18.1 mg. Patients improved on all efficacy measures within 1 week of treatment initiation. The mean improvement peaked at week 8 and was maintained through week 12 (MADRS, 18.5 (10.3)). | Memantine demonstrated early-onset efficacy in patients with major depression. | 2 |
| Muhonen et al. [29] | 2008 | RCT | 58 | Alcohol-dependentoutpatients with major depressivedisorder | In this 26-week study patients were treated with memantine (n = 29; 20 mg/day) or escitalopram (n = 29; 20 mg/day). Outcome measures were MADRS and Beck Depression Inventory-II for depression, Hamilton Rating Scale for Anxiety (HAM-A) and Beck Anxiety Inventory for anxiety, Consortium to Establish a Registry for Alzheimer’s Disease test battery for cognitive functions, and Social and Occupational Functioning Assessment Scale for social and occupational functions and quality-of-life measures. | Both treatments significantly reduced the baseline level of depression and anxiety according to MADRS and HAM-A, which were the primary measures (p < 0.0001). There was no significant difference between the memantine and escitalopram groups. Quality-of-life outcomes equally improved in both treatment groups. | These data provide new evidence for the safety and potential efficacy of memantine for major depressive disorder in patients with comorbid alcohol dependence. | 1 |
| Anand et al. [13] | 2012 | RCT | 29 | Subjects with depressive phase of bipolar disorder | Subjects on a stable dose of lamotrigine (100 mg or more) were randomized to either memantine (starting dose of 5 mg increased up to 20 mg over four weeks, then 20 mg stable dose from four to eight weeks) or matching pill placebo for eight weeks.Patients were rated on the 17-item HDRS and other behavioral measures weekly. | The 8-week repeated-measures mixed-effect model for HDRS was not significant for memantine vs. the placebo. Exploratory mixed-effect analyses for the first 4 weeks while the memantine dose was being titrated up every week revealed a significant decrease in HDRS scores from baseline (p = 0.007). | No statistically significant benefit of memantine augmentation of lamotrigine for patients with depressive phase of bipolar disorder over eight weeks was demonstrated. However, memantine had an antidepressant effect early on in the treatment while its dose was being titrated up. | 1 |
| Smith et al. [30] | 2013 | RCT | 31 | Subjects with majordepressivedisorder | Subjects were randomized to add memantine to current antidepressant treatment (flexible dose 5–20 mg/d, with all memantine group participants reaching the dose of 20 mg/d) (n = 15) or placebo (n = 16) to their existing treatment for 8 weeks. Primary outcome was measured by MADRS. Secondary outcomes included other depression and anxiety rating scales, suicidal and delusional ideation, and other adverse effects. | Participants receiving memantine did not show a statistically or clinically significant change in MADRS scores compared with the placebo over the entire study. Similarly, no substantial effect sizes favoring memantine, nor statistically significant between-group differences, were observed for the secondary efficacy outcomes. | This trial did not detect significant statistical or effect size differences between memantine and placebo augmentation among non-responders or poor responders to conventional antidepressants. | 1 |
| Strzelecki et al. [15] | 2013 | case report | 1 | 49-year-old male with manic moderate depressive episode | After an ineffective use of lithium, olanzapine and antidepressant treatment with mianserin, memantine was added up to 20 mg per day for 10 weeks. The mental state was assessed using the HDRS, the Young Mania Rating Scale (YMRS), the HAM-A, the Clinical Global Inventory, the World Health Organization Quality of Life Scale and psychological tests. | After 10 weeks, the patient achieved a partial symptomatic improvement in mood, anxiety, and quality of sleep, but his activity remained insufficient. We also observed an improvement in the parameters of cognitive functioning and quality of life. | Using memantine in bipolar depression may improve mood, cognitive functioning, and quality of life. | 3 |
| Stevens et al. [16] | 2013 | RCT | 29 | Bipolar depression patients | Patients on a stable dose of lamotrigine were randomized 1:1 to receive memantine 20 mg/day and placebo. The study lasted 4–8 weeks in the memantine group and 8 weeks in the placebo group. The severity of depression was measured with the HDRS-17 scale. | There were no differences in change in the HDRS-17 score between the groups. In the group treated with memantine, an acceleration of speed of response in HDRS-17 was shown. 12 patients improved in memantine group and 6 in placebo group. No relapses were observed in the memantine group. | Memantine added to lamotrigine caused an increased speed of response compared with placebo in bipolar depression patients. | 1 |
| Omranifard et al. [31] | 2014 | RCT | 57 | Subjects with depression | Memantine (20 mg daily) or identical placebo plus citalopram for 8 weeks. Severity of depression and quality of life was evaluated using the Geriatric Depression Scale (GDS-15) HDRS and World Health Organization Quality of Life WHOQOL-BREF, respectively. | The scores of the GDS-15, HRSD, and WHO-QOL-BREF scales at baseline, 4 weeks, and 8 weeks after initiating the trial did not change significantly after the use of memantine (p > 0.05). There was no significant difference in mean ± SD of GDS-15, HRSD, and WHO-QOL-BREF scales among the intervention and placebo groups (p > 0.05). | The outcome of this clinical trial did not support the antidepressant effect of add-on memantine in elderly patients with depression receiving citalopram. | 1 |
| Lee et al. [14] | 2014 | RCT | 232 | Subjects with bipolardisorder II depression | During the 12-week study, patients undergoing regular valproic acid treatments were randomly assigned to a group: valproic acid + memantine (5 mg/day) (n = 115) or valproic acid + placebo (n = 117). The HDRS and YMRS were used to evaluate the clinical responses during weeks 0, 1, 2, 4, 8, and 12. The genotypes of the brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms were determined using polymerase chain reactions plus restriction fragment length polymorphism analysis. | Both groups showed significantly decreased YMRS and HDRS scores after 12 weeks of treatment; the differences between groups were non-significant. When stratified by the BDNF Val66Met genotypes, significantly greater decreases in HDRS scores were found in the VPA + memantine group in patients with the Val Met genotype (p = 0.004). | The effectiveness of memantine in the treatment of depression depended on polymorphisms in the BDNF gene. | 1 |
| Amidfar et al. [32] | 2017 | RCT | 66 | Subjects with moderate-to-severe major depressivedisorder | 6 weeks of treatment with either memantine (20 mg/day) plus sertraline (200 mg/day) or placebo plus sertraline (200 mg/day). Patients were evaluated using HDRS at baseline and at weeks 2, 4, and 6. | A significantly greater improvement was seen at all three follow-up sessions, as well as significantly greater response rates at weeks 4 and 6, in the memantine group. Significantly more early improvements and more rapid response to treatment were observed in the memantine group. A significant reduction was observed in the HDRS score from baseline to the study endpoint in both the memantine and placebo groups. | A 6-week course of treatment with memantine as an adjunct to sertraline showed a favorable safety and efficacy profile in patients with major depressive disorder. | 1 |
| Lavretsky et al. [33] | 2020 | RCT | 62 | Older adults with depression and subjective memory complaints | Escitalopram + memantine (ESC/MEM) compared with escitalopram + placebo (ESC/PBO) for 6 months. The primary outcome was a change in depression, as assessed by HDRS post-treatment (at 6 months). Remission was defined as HDRS ≤6; naturalistic follow-up continued until 12 months. | The mean daily escitalopram dose was 11.1 mg (SD = 3.7; range: 5–20 mg). The mean daily memantine dose was 19.3 mg (SD = 2.6; range 10–20 mg). The remission rate within ESC/MEM was 45.8% and 47.9%, compared with 38.3% and 31.9% in ESC/PBO, at 3 and 6 months, respectively. Both groups improved significantly on the HAM-D at 3, 6, and 12 months, with no observed between-group differences. ESC/MEM demonstrated greater improvement in delayed recall and executive functioning at 12 months compared with ESC/PBO. | The combination of memantine with escitalopram was well tolerated and was as effective as escitalopram and placebo in improving depression using HAM-D. The combination of memantine and escitalopram was significantly more effective than escitalopram and the placebo at improving cognitive outcomes at 12 months. | 1 |
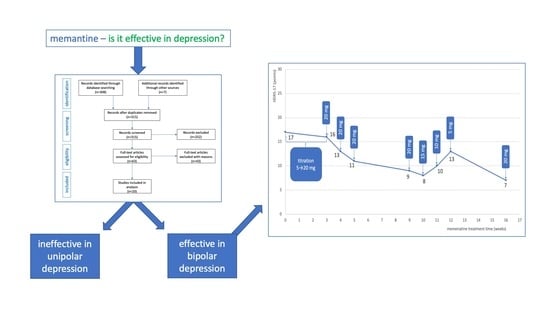
| Visit Time | HDRS-17 (Points) | Treatment (Daily Dose) |
|---|---|---|
| 11/2018 | 18 | quetiapine 300 mg |
| 01/2019 | 18 | quetiapine 300 mg + lamotrigine 150 mg |
| 03/2019 | 19 | lamotrigine 150 mg + aripiprazol 7.5 mg |
| 05/2019 | 18 | lamotrigine 150 mg + aripiprazol 7.5 mg |
| 07/2019 | 19 | lamotrigine 150 mg + valproic acid 1500 mg |
| 10/2019 | 18 | lamotrigine 150 mg + valproic acid 1500 mg + amantadine 100 mg/200 mg |
| 10/2019 | 15 | lamotrigine 150 mg + valproic acid 1500 mg + amantadine 200 mg |
| 11/2019 | 17 | lamotrigine 150 mg + valproic acid 1500 mg + memantine titrated-up every 3 days (5 mg/10 mg/15 mg/20 mg) |
| 11/2019 | 16 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 20 mg |
| 12/2019 | 13 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 20 mg |
| 12/2019 | 11 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 20 mg |
| 01/2020 | 9 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 20 mg |
| 02/2020 | 13 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 5 mg |
| 03/2020 | 7 | lamotrigine 150 mg + valproic acid 1500 mg + memantine 10 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystanek, M.; Surma, S.; Pałasz, A.; Romańczyk, M.; Krysta, K. Possible Antidepressant Effects of Memantine—Systematic Review with a Case Study. Pharmaceuticals 2021, 14, 481. https://doi.org/10.3390/ph14050481
Krzystanek M, Surma S, Pałasz A, Romańczyk M, Krysta K. Possible Antidepressant Effects of Memantine—Systematic Review with a Case Study. Pharmaceuticals. 2021; 14(5):481. https://doi.org/10.3390/ph14050481
Chicago/Turabian StyleKrzystanek, Marek, Stanisław Surma, Artur Pałasz, Monika Romańczyk, and Krzysztof Krysta. 2021. "Possible Antidepressant Effects of Memantine—Systematic Review with a Case Study" Pharmaceuticals 14, no. 5: 481. https://doi.org/10.3390/ph14050481
APA StyleKrzystanek, M., Surma, S., Pałasz, A., Romańczyk, M., & Krysta, K. (2021). Possible Antidepressant Effects of Memantine—Systematic Review with a Case Study. Pharmaceuticals, 14(5), 481. https://doi.org/10.3390/ph14050481