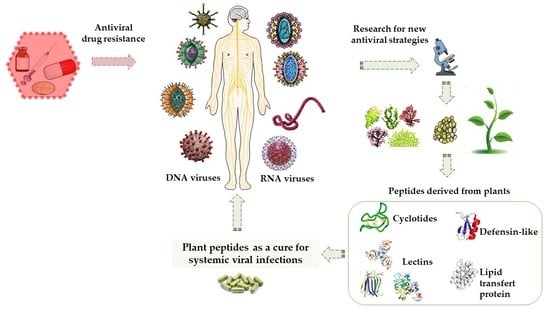
Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections
Abstract
:1. Introduction
3. Plant-Derived Antiviral Peptides against RNA Viruses
3.1. Human Immunodeficiency Virus (HIV)
3.2. Influenza Strain A H1N1
3.3. Junin Hemorrhagic Fever Virus (JHFV)
3.4. Respiratory Syncytial Virus (RSV)
3.5. Flavivirus (FV)
3.5.1. Yellow Fever Virus (YFV)
3.5.2. Dengue Virus (DENV)
3.5.3. Japan Encephalitis Virus (JEV)
3.5.4. Tick-Borne Encephalitis Virus (TBEV)
3.5.5. Zika Virus (ZIKV)
3.5.6. Chikungunya Virus (CHIKV)
3.5.7. Hepatitis C Virus (HCV)
3.6. Infectious Bronchitis Virus (IBV)
3.7. Foot and Mouth Disease Virus (FMDV) and Coxsackie Virus
3.8. Ebola Virus
3.9. Coronavirus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeter, R.G.; Khanderia, U. Recent Advances in Antiviral Therapy. Clin. Pharm. 1986, 5, 961–976. [Google Scholar] [PubMed]
- Whitley, R.; Alford, C.; Hess, F.; Buchanan, R. Vidarabine: A Preliminary Review of Its Pharmacological Properties and Therapeutic Use. Drugs 1980, 20, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Whitley, R. Neonatal Herpes Simplex Virus Infections: Where Are We Now? Adv. Exp. Med. Biol. 2011, 697, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, G. Acyclovir--and Beyond. J. Int. Med. Res. 1994, 22 (Suppl. 1), 33A–42A. [Google Scholar] [PubMed]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa Fever. Effective Therapy with Ribavirin. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Chung, R.T. Overview of Direct-Acting Antiviral Drugs and Drug Resistance of Hepatitis C Virus. Methods Mol. Biol. 2019, 1911, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Wills, M.R.; Sinclair, J.H. Advances in the Treatment of Cytomegalovirus. Br. Med. Bull. 2019, 131, 5–17. [Google Scholar] [CrossRef]
- Chen, S.-J.; Wang, S.-C.; Chen, Y.-C. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef]
- Weber, I.T.; Harrison, R.W. Tackling the Problem of HIV Drug Resistance. Postepy Biochem. 2016, 62, 273–279. [Google Scholar] [PubMed]
- Pawlotsky, J.-M. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagaglio, S.; Uberti-Foppa, C.; Morsica, G. Resistance Mechanisms in Hepatitis C Virus: Implications for Direct-Acting Antiviral Use. Drugs 2017, 77, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral Peptides as Promising Therapeutic Drugs. Cell Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, J.; Craik, D.J. Discovery, Structure, Function, and Applications of Cyclotides: Circular Proteins from Plants. J. Exp. Bot. 2016, 67, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Craik, D.J.; Du, J. Cyclotides as Drug Design Scaffolds. Curr. Opin. Chem. Biol. 2017, 38, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Natural Antimicrobial Peptides as Promising Anti-HIV Candidates. Curr. Top. Pept. Protein Res. 2012, 13, 93–110. [Google Scholar]
- Ishaq, N.; Bilal, M.; Iqbal, H.M.N. Medicinal Potentialities of Plant Defensins: A Review with Applied Perspectives. Medicines 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C.F. Lectins with Anti-HIV Activity: A Review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Marine Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, M.D.; Winter, H.C.; Goldstein, I.J.; Markovitz, D.M. A Lectin Isolated from Bananas Is a Potent Inhibitor of HIV Replication. J. Biol. Chem. 2010, 285, 8646–8655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbeau, P.; Haran, M.; Binz, H.; Devaux, C. Jacalin, a Lectin with Anti-HIV-1 Properties, and HIV-1 Gp120 Envelope Protein Interact with Distinct Regions of the CD4 Molecule. Mol. Immunol. 1994, 31, 569–575. [Google Scholar] [CrossRef]
- Witvrouw, M.; Fikkert, V.; Hantson, A.; Pannecouque, C.; O’keefe, B.R.; McMahon, J.; Stamatatos, L.; de Clercq, E.; Bolmstedt, A. Resistance of Human Immunodeficiency Virus Type 1 to the High-Mannose Binding Agents Cyanovirin N and Concanavalin A. J. Virol. 2005, 79, 7777–7784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, C.; He, X.; Niu, K.; Peng, H.; Li, W.; Zhou, C.; Bao, J. Molecular Modeling, Docking and Dynamics Simulations of GNA-Related Lectins for Potential Prevention of Influenza Virus (H1N1). J. Mol. Model. 2012, 18, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Charan, R.D.; Munro, M.H.; O’Keefe, B.R.; Sowder, R.; McKee, T.C.; Currens, M.J.; Pannell, L.K.; Boyd, M.R. Isolation and Characterization of Myrianthus Holstii Lectin, a Potent HIV-1 Inhibitory Protein from the Plant Myrianthus Holstii(1). J. Nat. Prod. 2000, 63, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Armand-Ugon, M.; Bastida, J.; Viladomat, F.; Esté, J.A.; Stewart, D.; Codina, C. Anti-Human Immunodeficiency Virus Type 1 (HIV-1) Activity of Lectins from Narcissus Species. Planta Med. 2003, 69, 109–112. [Google Scholar] [CrossRef]
- Ding, J.; Bao, J.; Zhu, D.; Zhang, Y.; Wang, D.-C. Crystal Structures of a Novel Anti-HIV Mannose-Binding Lectin from Polygonatum Cyrtonema Hua with Unique Ligand-Binding Property and Super-Structure. J. Struct. Biol. 2010, 171, 309–317. [Google Scholar] [CrossRef]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High Mannose-Binding Lectin with Preference for the Cluster of Alpha1-2-Mannose from the Green Alga Boodlea Coacta Is a Potent Entry Inhibitor of HIV-1 and Influenza Viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Cui, T.; Lam, Y. Synthesis and Disulfide Bond Connectivity-Activity Studies of a Kalata B1-Inspired Cyclopeptide against Dengue NS2B-NS3 Protease. Bioorg. Med. Chem. 2010, 18, 1331–1336. [Google Scholar] [CrossRef]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin Carrageenan Fast Dissolving Inserts Prevent SHIV HSV-2 and HPV Infections in Vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef] [PubMed]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination To Target Herpes Simplex Virus 2 and Human Papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-Inactivating Protein, from the Red Alga Griffithsia Sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum In Vitro Activity and In Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [Green Version]
- Ishag, H.Z.A.; Li, C.; Wang, F.; Mao, X. Griffithsin Binds to the Glycosylated Proteins (E and PrM) of Japanese Encephalitis Virus and Inhibit Its Infection. Virus Res. 2016, 215, 50–54. [Google Scholar] [CrossRef]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M.; et al. Antiviral Lectins from Red and Blue-Green Algae Show Potent in Vitro and in Vivo Activity against Hepatitis C Virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East Respiratory Syndrome Coronavirus Infection Is Inhibited by Griffithsin. Antiviral Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Limenin, a Defensin-like Peptide with Multiple Exploitable Activities from Shelf Beans. J. Pept. Sci. 2006, 12, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 1 May 2021).
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human Herpes Simplex Virus: Life Cycle and Development of Inhibitors. Biochem. Moscow 2014, 79, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Farr Zuend, C.; Nomellini, J.F.; Smit, J.; Horwitz, M.S. Generation of a Dual-Target, Safe, Inexpensive Microbicide That Protects Against HIV-1 and HSV-2 Disease. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebo, O.A. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.E.; Badillo-Corona, J.A.; Ramírez-Sotelo, G.; Oliver-Salvador, C. Biologically Active and Antimicrobial Peptides from Plants. Biomed. Res. Int. 2015, 2015, 102129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo Filho, I.; Cortez, D.A.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Antiviral Activity and Mode of Action of a Peptide Isolated from Sorghum Bicolor. Phytomedicine 2008, 15, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Dadé, M.; Zeinsteger, P.; Bozzolo, F.; Mestorino, N. Repellent and Lethal Activities of Extracts From Fruits of Chinaberry (Melia Azedarach L., Meliaceae) Against Triatoma Infestans. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Alché, L.E.; Barquero, A.A.; Sanjuan, N.A.; Coto, C.E. An Antiviral Principle Present in a Purified Fraction from Melia Azedarach L. Leaf Aqueous Extract Restrains Herpes Simplex Virus Type 1 Propagation. Phytother. Res. 2002, 16, 348–352. [Google Scholar] [CrossRef]
- Alché, L.E.; Berra, A.; Veloso, M.J.; Coto, C.E. Treatment with Meliacine, a Plant Derived Antiviral, Prevents the Development of Herpetic Stromal Keratitis in Mice. J. Med. Virol. 2000, 61, 474–480. [Google Scholar] [CrossRef]
- Barquero, A.A.; Alché, L.E.; Coto, C.E. Antiviral Activity of Meliacine on the Replication of a Thymidine Kinase-Deficient Mutant of Herpes Simplex Virus Type 1 Alone and in Combination with Acyclovir. Int. J. Antimicrob. Agents 1997, 9, 49–55. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal Plants: Treasure for Antiviral Drug Discovery. Phytother. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Petrera, E.; Coto, C.E. Effect of Meliacine, a Plant Derived Antiviral, on Tumor Necrosis Factor Alpha. Fitoterapia 2003, 74, 77–83. [Google Scholar] [CrossRef]
- Castilla, V.; Barquero, A.A.; Mersich, S.E.; Coto, C.E. In Vitro Anti-Junin Virus Activity of a Peptide Isolated from Melia Azedarach L. Leaves. Int. J. Antimicrob. Agents 1998, 10, 67–75. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Castilla, V.; Coto, C.E. Inhibition of Foot and Mouth Disease Virus (FMDV) Uncoating by a Plant-Derived Peptide Isolated from Melia Azedarach L. Leaves. Arch. Virol. 1998, 143, 581–590. [Google Scholar] [CrossRef]
- Henriques, S.T.; Huang, Y.-H.; Rosengren, K.J.; Franquelim, H.G.; Carvalho, F.A.; Johnson, A.; Sonza, S.; Tachedjian, G.; Castanho, M.A.R.B.; Daly, N.L.; et al. Decoding the Membrane Activity of the Cyclotide Kalata B1. J. Biol. Chem. 2011, 286, 24231–24241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, N.L.; Clark, R.J.; Plan, M.R.; Craik, D.J. Kalata B8, a Novel Antiviral Circular Protein, Exhibits Conformational Flexibility in the Cystine Knot Motif. Biochem. J. 2006, 393, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Vulgarinin, a Broad-Spectrum Antifungal Peptide from Haricot Beans (Phaseolus Vulgaris). Int. J. Biochem. Cell. Biol. 2005, 37, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Lunatusin, a Trypsin-Stable Antimicrobial Peptide from Lima Beans (Phaseolus Lunatus L.). Peptides 2005, 26, 2086–2092. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. Cicerin and Arietin, Novel Chickpea Peptides with Different Antifungal Potencies. Peptides 2002, 23, 817–822. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. Isolation of a New Cyclophilin-like Protein from Chickpeas with Mitogenic, Antifungal and Anti-HIV-1 Reverse Transcriptase Activities. Life Sci. 2002, 70, 1129–1138. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. An Antifungal Peptide from Baby Lima Bean. Appl. Microbiol. Biotechnol. 2006, 73, 576–581. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. An Antifungal Peptide from the Coconut. Peptides 2005, 26, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; Sowder, R.C.; Henderson, L.E.; Parsons, I.C.; Kashman, Y.; Cardellina, J.H.; McMahon, J.B.; Buckheit, R.W.; Pannell, L.K.; Boyd, M.R. Circulins A and B. Novel Human Immunodeficiency Virus (HIV)-Inhibitory Macrocyclic Peptides from the Tropical Tree Chassalia Parvifolia. J. Am. Chem. Soc. 1994, 116, 9337–9338. [Google Scholar] [CrossRef]
- Lin, P.; Ng, T.B. Preparation and Biological Properties of a Melibiose Binding Lectin from Bauhinia Variegata Seeds. J. Agric. Food Chem. 2008, 56, 10481–10486. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Renders, M.; Férir, G.; Huskens, D.; Van Damme, E.J.M.; Peumans, W.; Balzarini, J.; Schols, D. NICTABA and UDA, Two GlcNAc-Binding Lectins with Unique Antiviral Activity Profiles. J. Antimicrob. Chemother. 2015, 70, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-Z.; Yang, Y.; Zhang, S.-X.; Tang, L.; Wang, H.-M.; Chen, C.-J.; Shen, Z.-F.; Cheng, K.-D.; Kong, J.-Q.; Wang, W. A cyclotide against influenza A H1N1 virus from Viola yedoensis. Yao Xue Xue Bao Acta Pharm. Sin. 2014, 49, 905–912. [Google Scholar]
- Maximiano, M.R.; Franco, O.L. Biotechnological Applications of Versatile Plant Lipid Transfer Proteins (LTPs). Peptides 2021, 140, 170531. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.M.; Tian, L.; Su, M.; Ho, W.-S.; Sun, S.S.M.; Chung, H.-Y.; Wong, H.N.C.; Ooi, V.E.C. Isolation, Characterization, Molecular Cloning and Modeling of a New Lipid Transfer Protein with Antiviral and Antiproliferative Activities from Narcissus Tazetta. Peptides 2008, 29, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.M.; Ho, W.-S.; Ngai, K.L.K.; Tian, L.; Chan, P.K.S.; Sun, S.S.M.; Ooi, V.E.C. Narcissus Tazetta Lectin Shows Strong Inhibitory Effects against Respiratory Syncytial Virus, Influenza A (H1N1, H3N2, H5N1) and B Viruses. J. Biosci. 2010, 35, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Krobthong, S.; Paemanee, A.; Roytrakul, S.; Rattanabunyong, S.; Choowongkomon, K.; Yenchitsomanus, P.-T. Novel Bioactive Peptides Demonstrating Anti-Dengue Virus Activity Isolated from the Asian Medicinal Plant Acacia Catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.T.; Ooi, J.S.G.; Nguyen, N.T.K.; Wang, S.; Huang, M.; Liu, D.X.; Tam, J.P. Antiviral Cystine Knot α-Amylase Inhibitors from Alstonia Scholaris. J. Biol. Chem. 2015, 290, 31138–31150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthasarathy, A.; Borrego, E.J.; Savka, M.A.; Dobson, R.C.J.; Hudson, A.O. Amino Acid-Derived Defense Metabolites from Plants: A Potential Source to Facilitate Novel Antimicrobial Development. J. Biol. Chem. 2021, 100438. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Mudgal, R.; Jose, J.; Tomar, S. Glycan-Dependent Chikungunya Viral Infection Divulged by Antiviral Activity of NAG Specific Chi-like Lectin. Virology 2019, 526, 91–98. [Google Scholar] [CrossRef]
- Xu, X.-C.; Zhang, Z.-W.; Chen, Y.-E.; Yuan, M.; Yuan, S.; Bao, J.-K. Antiviral and Antitumor Activities of the Lectin Extracted from Aspidistra Elatior. Zeitschrift Naturforschung C 2015, 70, 7–13. [Google Scholar] [CrossRef]
- Covés-Datson, E.M.; Dyall, J.; DeWald, L.E.; King, S.R.; Dube, D.; Legendre, M.; Nelson, E.; Drews, K.C.; Gross, R.; Gerhardt, D.M.; et al. Inhibition of Ebola Virus by a Molecularly Engineered Banana Lectin. PLoS Negl. Trop. Dis. 2019, 13, e0007595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sanjosé, S.; Diaz, M.; Castellsagué, X.; Clifford, G.; Bruni, L.; Muñoz, N.; Bosch, F.X. Worldwide Prevalence and Genotype Distribution of Cervical Human Papillomavirus DNA in Women with Normal Cytology: A Meta-Analysis. Lancet Infect. Dis. 2007, 7, 453–459. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef]
- Araújo, M.G.; Magalhães, G.M.; Garcia, L.C.; Vieira, É.C.; Carvalho-Leite, M.D.L.R.D.; Guedes, A.C.M. Update on Human Papillomavirus—Part II: Complementary Diagnosis, Treatment and Prophylaxis. An. Bras. Dermatol. 2021, 96, 125–138. [Google Scholar] [CrossRef]
- Vanangamudi, M.; Nair, P.C.; Engels, S.E.M.; Palaniappan, S.; Namasivayam, V. Structural Insights to Human Immunodeficiency Virus (HIV-1) Targets and Their Inhibition. Adv. Exp. Med. Biol. 2021, 1322, 63–95. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 11 June 2021).
- Maeda, K.; Das, D.; Kobayakawa, T.; Tamamura, H.; Takeuchi, H. Discovery and Development of Anti-HIV Therapeutic Agents: Progress Towards Improved HIV Medication. Curr. Top. Med. Chem. 2019, 19, 1621–1649. [Google Scholar] [CrossRef] [PubMed]
- Grover, T.; Mishra, R.; Gulati, P.; Mohanty, A. An Insight into Biological Activities of Native Cyclotides for Potential Applications in Agriculture and Pharmaceutics. Peptides 2021, 135, 170430. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.C.; Wang, C.K.L.; Gustafson, K.R.; Craik, D.J. Cyclotides as Natural Anti-HIV Agents. Biopolymers 2008, 90, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, S.T.; Craik, D.J. Cyclotides as Templates in Drug Design. Drug Discov. Today 2010, 15, 57–64. [Google Scholar] [CrossRef]
- Ngai, P.H.K.; Ng, T.B. Phaseococcin, an Antifungal Protein with Antiproliferative and Anti-HIV-1 Reverse Transcriptase Activities from Small Scarlet Runner Beans. Biochem. Cell Biol. 2005, 83, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Xia, L.; Wong, J.H.; Ng, T.B.; Ye, X.; Wang, S.; Shi, X. Lipid Transfer Proteins from Brassica Campestris and Mung Bean Surpass Mung Bean Chitinase in Exploitability. J. Pept. Sci. 2007, 13, 642–648. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B. Sesquin, a Potent Defensin-like Antimicrobial Peptide from Ground Beans with Inhibitory Activities toward Tumor Cells and HIV-1 Reverse Transcriptase. Peptides 2005, 26, 1120–1126. [Google Scholar] [CrossRef]
- Lin, P.; Wong, J.H.; Ng, T.B. A Defensin with Highly Potent Antipathogenic Activities from the Seeds of Purple Pole Bean. Biosci. Rep. 2009, 30, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.H.; Ng, T.B. Gymnin, a Potent Defensin-like Antifungal Peptide from the Yunnan Bean (Gymnocladus Chinensis Baill). Peptides 2003, 24, 963–968. [Google Scholar] [CrossRef]
- Hansen, J.E.; Nielsen, C.M.; Nielsen, C.; Heegaard, P.; Mathiesen, L.R.; Nielsen, J.O. Correlation between Carbohydrate Structures on the Envelope Glycoprotein Gp120 of HIV-1 and HIV-2 and Syncytium Inhibition with Lectins. AIDS 1989, 3, 635–641. [Google Scholar] [CrossRef]
- Hu, B.; Du, T.; Li, C.; Luo, S.; Liu, Y.; Huang, X.; Hu, Q. Sensitivity of Transmitted and Founder Human Immunodeficiency Virus Type 1 Envelopes to Carbohydrate-Binding Agents Griffithsin, Cyanovirin-N and Galanthus Nivalis Agglutinin. J. Gen. Virol. 2015, 96, 3660–3666. [Google Scholar] [CrossRef]
- Russell, C.J. Hemagglutinin Stability and Its Impact on Influenza A Virus Infectivity, Pathogenicity, and Transmissibility in Avians, Mice, Swine, Seals, Ferrets, and Humans. Viruses 2021, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- WHO. Pandemic (H1N1) 2009. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/pandemic-influenza/past-pandemics/pandemic-h1n1-2009 (accessed on 25 June 2021).
- Al Khatib, H.A.; Al Thani, A.A.; Gallouzi, I.; Yassine, H.M. Epidemiological and Genetic Characterization of PH1N1 and H3N2 Influenza Viruses Circulated in MENA Region during 2009–2017. BMC Infect. Dis. 2019, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T. Influenza and Antiviral Resistance: An Overview. Eur J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef]
- Grant, A.; Seregin, A.; Huang, C.; Kolokoltsova, O.; Brasier, A.; Peters, C.; Paessler, S. Junín Virus Pathogenesis and Virus Replication. Viruses 2012, 4, 2317–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Gao, Y.; Liang, B. Structural Insights into the Respiratory Syncytial Virus RNA Synthesis Complexes. Viruses 2021, 13, 834. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Tripp, R.A. Respiratory Syncytial Virus: Prospects for New and Emerging Therapeutics. Expert Rev. Respir. Med. 2017, 11, 609–615. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- RSV Vaccine Research and Development Technology Roadmap. Available online: https://www.who.int/publications-detail-redirect/WHO-IVB-17.12 (accessed on 25 June 2021).
- Goldstein, E.; Nguyen, H.H.; Liu, P.; Viboud, C.; Steiner, C.A.; Worby, C.J.; Lipsitch, M. On the Relative Role of Different Age Groups During Epidemics Associated With Respiratory Syncytial Virus. J. Infect. Dis. 2018, 217, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Simoes, E.A.; Carbonell-Estrany, X. Impact of Severe Disease Caused by Respiratory Syncytial Virus in Children Living in Developed Countries. Pediatr. Infect. Dis. J. 2003, 22, S13–S18; discussion S18–S20. [Google Scholar] [CrossRef]
- Fergie, J.; Goldstein, M.; Krilov, L.R.; Wade, S.W.; Kong, A.M.; Brannman, L. Update on Respiratory Syncytial Virus Hospitalizations among U.S. Preterm and Term Infants before and after the 2014 American Academy of Pediatrics Policy on Immunoprophylaxis: 2011-2017. Hum. Vaccin. Immunother. 2021, 17, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.; Tait, D.; Efthimiou, J.; Mori, J.; Kim, Y.-I.; Thomas, E.; Wilson, L.; Harland, R.; Mathews, N.; Cockerill, S.; et al. A Randomized, Placebo-Controlled, Respiratory Syncytial Virus Human Challenge Study of the Antiviral Efficacy, Safety, and Pharmacokinetics of RV521, an Inhibitor of the RSV-F Protein. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ark Biosciences Inc. A Randomised, Double-Blind., Placebo-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of Orally Administered AK0529 in Adults With Respiratory Syncytial Virus Infection; Ark Biosciences Inc.: Shanghai, China, 2019. [Google Scholar]
- RSV Vaccine and MAb Snapshot. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 16 June 2021).
- Contigiani, M.S.; Diaz, L.A.; Spinsanti, L. Flavivirus. In Arthropod Borne Diseases; Marcondes, C.B., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 73–88. ISBN 978-3-319-13884-8. [Google Scholar]
- Rodriguez, A.K.; Muñoz, A.L.; Segura, N.A.; Rangel, H.R.; Bello, F. Molecular Characteristics and Replication Mechanism of Dengue, Zika and Chikungunya Arboviruses, and Their Treatments with Natural Extracts from Plants: An Updated Review. EXCLI J. 2019, 18, 988–1006. [Google Scholar] [CrossRef] [PubMed]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-Spectrum Agents for Flaviviral Infections: Dengue, Zika and Beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.H.; Barrett, A.D.T. Structure-Function of the Yellow Fever Virus Envelope Protein: Analysis of Antibody Epitopes. Viral Immunol. 2020, 33, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yellow Fever—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/yellow-fever (accessed on 16 June 2021).
- Chen, L.H.; Wilson, M.E. Yellow Fever Control: Current Epidemiology and Vaccination Strategies. Trop. Dis. Travel Med. Vaccines 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C. Yellow Fever. In Arthropod Borne Diseases; Marcondes, C.B., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 101–113. ISBN 978-3-319-13884-8. [Google Scholar]
- Figueiredo, G.G.; Coronel, O.A.; Trabuco, A.C.; Bazán, D.E.; Russo, R.R.; Alvarenga, N.L.; Aquino, V.H. Steroidal Saponins from the Roots of Solanum Sisymbriifolium Lam. (Solanaceae) Have Inhibitory Activity against Dengue Virus and Yellow Fever Virus. Braz. J. Med. Biol. Res. 2021, 54, e10240. [Google Scholar] [CrossRef] [PubMed]
- Castilla, V.; Sepúlveda, C.S.; García, C.C.; Damonte, E.B. Progress for antiviral development in Latin America. In Human Virology in Latin America; Springer: Cham, Switzerland, 2017; pp. 439–460. [Google Scholar] [CrossRef]
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 23 May 2021).
- Poonsiri, T.; Wright, G.S.A.; Solomon, T.; Antonyuk, S.V. Crystal Structure of the Japanese Encephalitis Virus Capsid Protein. Viruses 2019, 11, 623. [Google Scholar] [CrossRef] [Green Version]
- Navyashree, V.; Kant, K.; Kumar, A. Natural Chemical Entities from Arisaema Genus Might Be a Promising Break-through against Japanese Encephalitis Virus Infection: A Molecular Docking and Dynamics Approach. J. Biomol. Struct. Dyn. 2021, 39, 1404–1416. [Google Scholar] [CrossRef]
- Pulkkinen, L.I.A.; Butcher, S.J.; Anastasina, M. Tick-Borne Encephalitis Virus: A Structural View. Viruses 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.Y.; Fibriansah, G.; Kostyuchenko, V.A.; Ng, T.-S.; Lim, X.-X.; Zhang, S.; Lim, X.-N.; Wang, J.; Shi, J.; Morais, M.C.; et al. Capsid Protein Structure in Zika Virus Reveals the Flavivirus Assembly Process. Nat. Commun. 2020, 11, 895. [Google Scholar] [CrossRef]
- Yap, M.L.; Klose, T.; Urakami, A.; Hasan, S.S.; Akahata, W.; Rossmann, M.G. Structural Studies of Chikungunya Virus Maturation. Proc. Natl. Acad. Sci. USA 2017, 114, 13703–13707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Deng, C.-L.; Li, J.-Q.; Li, N.; Zhang, Q.-Y.; Ye, H.-Q.; Yuan, Z.-M.; Zhang, B. Infectious Chikungunya Virus (CHIKV) with a Complete Capsid Deletion: A New Approach for a CHIKV Vaccine. J. Virol. 2019, 93, e00504-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozov, V.A.; Lagaye, S. Hepatitis C Virus: Morphogenesis, Infection and Therapy. World J. Hepatol. 2018, 10, 186–212. [Google Scholar] [CrossRef]
- Irshad, M.; Dhar, I. Hepatitis C Virus Core Protein: An Update on Its Molecular Biology, Cellular Functions and Clinical Implications. Med. Princ. Pract. 2006, 15, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, R.; Farshadpour, F. Global Elimination of Hepatitis C Virus Infection: Progresses and the Remaining Challenges. World J. Hepatol. 2017, 9, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 17 July 2021).
- Jackwood, M.W. Review of Infectious Bronchitis Virus around the World. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzo, G.; Legnardi, M.; Tucciarone, C.M.; Drigo, M.; Martini, M.; Cecchinato, M. Evolution of Infectious Bronchitis Virus in the Field after Homologous Vaccination Introduction. Vet. Res. 2019, 50, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bande, F.; Arshad, S.S.; Omar, A.R.; Hair-Bejo, M.; Mahmuda, A.; Nair, V. Global Distributions and Strain Diversity of Avian Infectious Bronchitis Virus: A Review. Anim. Health Res. Rev. 2017, 18, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, D. Coronavirus Avian Infectious Bronchitis Virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, B. Vaccination against Infectious Bronchitis Virus: A Continuous Challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef]
- Heydari, H.; Golmohammadi, R.; Mirnejad, R.; Tebyanian, H.; Fasihi-Ramandi, M.; Moosazadeh Moghaddam, M. Antiviral Peptides against Coronaviridae Family: A Review. Peptides 2021, 139, 170526. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, S.-Q.; Guo, H.-C. Biological Function of Foot-and-Mouth Disease Virus Non-Structural Proteins and Non-Coding Elements. Virol. J. 2016, 13, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubman, M.J.; Baxt, B. Foot-and-Mouth Disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, K.; Mahapatra, M.; Upadhyaya, S.; Paton, D.J.; Babu, A.; Hutchings, G.; Parida, S. Genetic and Antigenic Characterization of Serotype O FMD Viruses from East Africa for the Selection of Suitable Vaccine Strain. Vaccine 2017, 35, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.A.M.; Noda, T.; Riches, J.D.; Kraehling, V.; Kolesnikova, L.; Becker, S.; Kawaoka, Y.; Briggs, J.A.G. Structural Dissection of Ebola Virus and Its Assembly Determinants Using Cryo-Electron Tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4275–4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Fontela, C.; McElroy, A.K. Ebola Virus Disease in Humans: Pathophysiology and Immunity. Marburg Ebolaviruses 2017, 411, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, S.; Ebihara, H. Pathogenicity and Virulence of Ebolaviruses with Species- and Variant-Specificity. Virulence 2021, 12, 885–901. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, P.; Wilkins, E.; Woodhead, M. Severe Acute Respiratory Syndrome (SARS): A Review. Clin. Med. 2004, 4, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Beig Parikhani, A.; Bazaz, M.; Bamehr, H.; Fereshteh, S.; Amiri, S.; Salehi-Vaziri, M.; Arashkia, A.; Azadmanesh, K. The Inclusive Review on SARS-CoV-2 Biology, Epidemiology, Diagnosis, and Potential Management Options. Curr. Microbiol. 2021, 78, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Almutary, A.G.; Sukamaran, A.; Yang, B.T.W.; Lee, X.T.; Lim, W.X.; Ng, Y.M.; Ibrahim, R.; Darmarajan, T.; Nanjappan, S.; et al. Middle East Respiratory Syndrome (MERS) Virus-Pathophysiological Axis and the Current Treatment Strategies. AAPS PharmSciTech 2021, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Chattu, V.K.; Ranade, A.V.; Das, D.; Basavarajegowda, A.; Bukelo, M. A Rapid Review of Recent Advances in Diagnosis, Treatment and Vaccination for COVID-19. AIMS Public Health 2021, 8, 137–153. [Google Scholar] [CrossRef] [PubMed]

| Peptides | Plant Source | Virus Target | EC50 | IC50 | References |
|---|---|---|---|---|---|
| Cyclotides | Rubiaceae and Violaceae plant families | HIV, infuenza H1N1 and DENV—disruption of viral envelope | - | - | [13] [15] |
| Kalata B1-inspired peptide | Synthetic derivative of kalata B1 peptide | Anti-DENV NS2B–NS3 protease | NI | Isomer 1B: 4.3 μM Isomer 1C: 9.3 μM | [13] [28] |
| Griffithsin (GRFT) | Griffithsia sp (Montagne) | Anti-HIV, -MERS- CoV, -SARS-CoV, -hepatitis C virus (HCV), -HSV-2, -human papillomavirus (HPV), and anti-Japanese encephalitis virus (JEV) | SARS-CoV: 48 nM HSV2 EC90: 12 ng/mL MERS- CoV: 2 mg/mL JEV EC50: 100 µg/mL HCV: 0.4 nM HPV16: 1.39 μM HPV18: 0.428 μM HPV45: 0.928 μM | HIV:0.043–0.63 nM JEV: 20nM | [19] [29] [30] [31] [32] [33] [34] [35] |
| Peptide with sequence homology to defensins | Phaseolus vulgaris cv. | Reduction of the activity of HIV-1 reverse transcriptase | NI | 0.5 μM | [13] [20] |
| Banana lectin (BanLec) | Musa acuminate cultivars cv. Grand Nain | Anti-HIV activity | NI | 0.49–2.06 nM | [20] |
| Artocarpus heterophyllus (jacalin) lectin | Artocarpus heterophyllus Lam. | Anti-HIV activity | NI | NI | [21] |
| Galanthus nivalis (snowdrop) agglutinin-related lectins Yucca filamentosa lectin (YFL-I) | Galanthus nivalis L. | Anti-HIV activity; antiviral activity against influenza virus H1N1 | NI | HIV: >500 nM | [23] [36] |
| Myrianthus holstii lectin (MHL) | Myrianthus holstii Engl. | Anti-HIV activity | 150 nM | NI | [24] |
| Narcissus pseudonarcissus lectin (NPA) | Narcissus pseudonarcissusL. | Anti-HIV activity | 2.02 μg/mL | 53.7 nM | [25] |
| Polygonatum cyrtonema lectin (PCL) | Polygonatum cyrtonema (Hua) | Anti-HIV activity. | NI | NI | [26] |
| Boodlea coacta lectin (BCA) | Boodlea coacta (Dickie) | Anti-HIV activity; antiviral activity against influenza virus H1N1 | HIV: 8.2 nM H3N2: 18.8–74.2 nM H1N1: 79.3–1590.2 nM | NI | [27] |
| Phaseococcin | Phaseolus coccineus (Minor) | Inhibition of the reverse transcriptase activity of the HIV virus | NI | 150 μM | [10] [20] [22] [37] |
| Sesquin (defensin-like) | Vigna sesquipedaliscv. ‘Ground Bean’ | Inhibition the reverse transcriptase activity of the HIV virus | NI | 50–200 μM | [38] [39] |
| Limenin (defensin-like) | Phaseolus limensis cv. | Reduction of the activity of HIV-1 reverse transcriptase | NI | 106 µM | [40] |
| Gymnin | Gymnocladus chinensis Baill. | Inhibition of the reverse transcriptase activity of the HIV virus | NI | 200 μM | [41] |
| 2 kD peptide | Sorghum bicolor (L.) Moench | Inhibition of the replication of HSV-1 | 6.25 μM | 6.25 to 50 μM | [13] [40] [41] [42] |
| Meliacine | Melia azedarach L. | Antiviral action on HSV-1, Junin virus (JV), and foot and mouth disease virus (FMDV) | HSV: 0.82 μg/mL HSV TK−: 0.41 μg/mL JV: 0.13 μg/mL FMDV: 0.5 μg/mL | NI | [43] [44] [45] [46] [47] [48] [49] [50] |
| Canavalia ensiformis Lectin (concanavalin a) | Canavalia ensiformisL. | Anti-HIV activity | NI | NI | [48] |
| Pep-RTYM | Synthetic derived plant peptide | Anti-DENV activity | 1.25 µg/mL | [48] | |
| Kalata B1 | Oldenlandia affnis (Roem. & Schult.) | Anti-HIV activity by destroying the viral particles prior to cell entry and inhibiting fusion of the virus to the host membrane | 2.04 μM | NI | [51] |
| Kalata B8 | Oldenlandia affinis (Roem. & Schult.) | Anti-HIV activity, proposed to be membrane dependent | 2.5 μM | NI | [52] |
| Vulgarinin | Phaseolus vulgaris | Inhibition of HIV-1 reverse transcriptase | NI | 130 μM | [53] |
| Lunatusin | Phaseolus lunatus L. | Inhibition of HIV-1 reverse transcriptase | NI | 120 μM | [54] |
| Cicerin | Cicer arietinum L. | Anti-HIV-1 reverse transcriptase activity | NI | 200 μM | [55] |
| Arietin | Cicer arietinum L. | Anti-HIV-1 reverse transcriptase activity | NI | 200 μM | [55] |
| Cyclophilin-like | Cicer arietinum L. | Inhibition HIV-1 reverse transcriptase | NI | 20 μM | [56] |
| Brassica LTP | Brassica campestris ssp. chinensis | Inhibition of the activity of HIV-1 reverse transcriptase | NI | 4 µM | [57] [58] |
| Circulin A & B | Chassalia parvifolia (K. Schum.) | Anti-HIV activity | NI | 40–260 nM | [59] |
| Melibiose-binding lectin Bauhinia variegata lectin | Bauhinia variegate L. | Inhibition of the reverse transcriptase activity of the HIV virus | NI | 1.02 µM | [60] |
| Lectin (NICTABA) | Nicotiana tabacum var. Samsun NN | Anti-HIV activity | 0.023–0.28 mM | NI | [61] |
| Cycloviolacin VY1 and VY5 | Viola yedoensis (Makino) | Anti-HIV activity Anti-influenza A H1N1 virus | NI | 2.27 mg/mL | [62] |
| Fetuin-binding peptide (lipid transfer proteins-LTP) | Narcissus tazetta var.chinensis | Inhibition of RSV and the cytopathic effect induced by influenza A (H1N1) virus | H1N1: 4.47 μg/mL RSV: 50 μg/mL (final C) | NI | [63] [64] |
| Narcissus tazetta lectin [NTL] | Narcissus tazetta var.chinensis | Inhibition of RSV and the cytopathic effect induced by influenza A (H1N1) virus | RSV: 2.30 µg/mL H1N1: 0.20 µg/mL | NI | [65] |
| Peptide 2 and 4 | Acacia catechu (L.f.) Willd | Anti-DENV | 6.25 μM | 15.25 μM | [66] |
| Knottins Alstotides (As1) | Alstonia scholaris Linn. R. Br. | Inhibition of the early phase of infectious bronchitis (IBV) (binds to IBV spike) virus and DENV infection | 90 μM | NI | [67] [68] |
| Tamarindus indica lectin (TCLL) | Tamarindus indica L. | Anti-alphaviruses (chikungunya viral infection) | >100 µM | N | [69] |
| Lectins | Aspidistra elatior (Blume) | Antiviral action on Coxsackie virus B4 and RSV | 4 μg/mL | 100 μg/mL | [70] |
| Engineered banana lectin (BanLec) | Synthetic derived plant peptide | Antiviral activity against Ebola virus disease (EVD) | NI | 1–6 µM | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammari, N.; Krier, Y.; Albert, Q.; Devocelle, M.; Varbanov, M.; on behalf of the OEMONOM. Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections. Pharmaceuticals 2021, 14, 774. https://doi.org/10.3390/ph14080774
Mammari N, Krier Y, Albert Q, Devocelle M, Varbanov M, on behalf of the OEMONOM. Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections. Pharmaceuticals. 2021; 14(8):774. https://doi.org/10.3390/ph14080774
Chicago/Turabian StyleMammari, Nour, Ysaline Krier, Quentin Albert, Marc Devocelle, Mihayl Varbanov, and on behalf of the OEMONOM. 2021. "Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections" Pharmaceuticals 14, no. 8: 774. https://doi.org/10.3390/ph14080774
APA StyleMammari, N., Krier, Y., Albert, Q., Devocelle, M., Varbanov, M., & on behalf of the OEMONOM. (2021). Plant-Derived Antimicrobial Peptides as Potential Antiviral Agents in Systemic Viral Infections. Pharmaceuticals, 14(8), 774. https://doi.org/10.3390/ph14080774