Doxycycline Attenuates Cancer Cell Growth by Suppressing NLRP3-Mediated Inflammation
Abstract
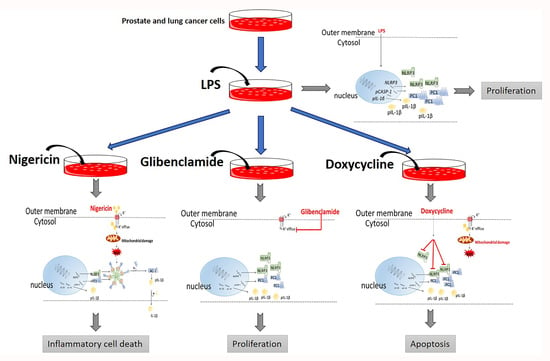
:1. Introduction
2. Results
2.1. Doxycycline Attenuates NLRP3 Inflammasome Activation
2.2. Doxycycline Decreased Cell Proliferation and Viability
2.3. The Effect of Doxycycline on Cytokine Secretion
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Maintenance
4.2. Reagents
4.3. Real Time-qPCR
4.4. Western Blot
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Real-Time Cell Proliferation Assay
4.7. Annexin V Assay
4.8. Cytokine Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010, 285, 6477–6488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, R.; Liu, G.H.; Janowski, A.M.; Sutterwala, F.S.; Zhang, W. Inflammasomes in cancer: A double-edged sword. Protein Cell 2014, 5, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, J. Inflammasomes in Inflammation-Induced Cancer. Front. Immunol. 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020, 11, 1444. [Google Scholar] [CrossRef]
- Balahura, L.R.; Selaru, A.; Dinescu, S.; Costache, M. Inflammation and Inflammasomes: Pros and Cons in Tumorigenesis. J. Immunol. Res. 2020, 2020, 2549763. [Google Scholar] [CrossRef]
- Tezcan, G.; Martynova, E.V.; Gilazieva, Z.E.; McIntyre, A.; Rizvanov, A.A.; Khaiboullina, S.F. MicroRNA Post-transcriptional Regulation of the NLRP3 Inflammasome in Immunopathologies. Front. Pharmacol. 2019, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Tezcan, G.; Garanina, E.E.; Alsaadi, M.; Gilazieva, Z.E.; Martinova, E.V.; Markelova, M.I.; Arkhipova, S.S.; Hamza, S.; McIntyre, A.; Rizvanov, A.A.; et al. Therapeutic Potential of Pharmacological Targeting NLRP3 Inflammasome Complex in Cancer. Front. Immunol. 2021, 11, 607881. [Google Scholar] [CrossRef]
- Tengesdal, I.W.; Menon, D.R.; Osborne, D.G.; Neff, C.P.; Powers, N.E.; Gamboni, F.; Mauro, A.G.; D’Alessandro, A.; Stefanoni, D.; Henen, M.A.; et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc. Natl. Acad. Sci. USA 2021, 118, e2000915118. [Google Scholar] [CrossRef] [PubMed]
- Kamp, D.W.; Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer: The role of the mitochondria. Oncology (Williston Park, N.Y.) 2011, 25, 400–413. [Google Scholar]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J.D.; Balin, B.J.; Appelt, D.M.; Sassi-Gaha, S.; Purohit, M.; Rest, R.F.; Artlett, C.M. NLRP3 inflammasome is a target for development of broad-spectrum anti-infective drugs. Antimicrob. Agents Chemother. 2012, 56, 1921–1930. [Google Scholar] [CrossRef] [Green Version]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Allam, R.; Darisipudi, M.N.; Rupanagudi, K.V.; Lichtnekert, J.; Tschopp, J.; Anders, H.J. Cutting edge: Cyclic polypeptide and aminoglycoside antibiotics trigger IL-1β secretion by activating the NLRP3 inflammasome. J. Immunol. 2011, 186, 2714–2718. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Hernández, D.L. Letter to the Editor: Use of Antibiotics, Gut Microbiota, and Risk of Type 2 Diabetes: Epigenetics Regulation. J. Clin. Endocrinol. Metab. 2016, 101, L62–L63. [Google Scholar] [CrossRef]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Fife, R.S.; Rougraff, B.T.; Proctor, C.; Sledge, G.W., Jr. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J. Lab. Clin. Med. 1997, 130, 530–534. [Google Scholar] [CrossRef]
- Fife, R.S.; Sledge, G.W., Jr.; Roth, B.J.; Proctor, C. Effects of doxycycline on human prostate cancer cells in vitro. Cancer Lett. 1998, 127, 37–41. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Govi, S.; Pasini, E.; Mappa, S.; Bertoni, F.; Zaja, F.; Montalbán, C.; Stelitano, C.; Cabrera, M.E.; Giordano Resti, A.; et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: Final results of an international phase II trial. J. Clin. Oncol. 2012, 30, 2988–2994. [Google Scholar] [CrossRef] [Green Version]
- Han, J.J.; Kim, T.M.; Jeon, Y.K.; Kim, M.K.; Khwarg, S.I.; Kim, C.W.; Kim, I.H.; Heo, D.S. Long-term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B cell lymphoma. Ann. Hematol. 2015, 94, 575–581. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, X.; Wu, D.; Jin, X.; Liu, R.; Hu, X.; Fu, Y.; Ding, Z.; Zhang, N.; Cao, Y. Doxycycline Attenuates Leptospira-Induced IL-1β by Suppressing NLRP3 Inflammasome Priming. Front. Immunol. 2017, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Mueller, J.L.; Vitari, A.C.; Misaghi, S.; Fedorova, A.; Deshayes, K.; Lee, W.P.; Hoffman, H.M.; Dixit, V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009, 187, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsvari, B.; Sotgia, F.; Lisanti, M.P. A new mutation-independent approach to cancer therapy: Inhibiting oncogenic RAS and MYC, by targeting mitochondrial biogenesis. Aging 2017, 9, 2098–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017, 8, 67269–67286. [Google Scholar] [CrossRef] [Green Version]
- Nadler, R.B.; Humphrey, P.A.; Smith, D.S.; Catalona, W.J.; Ratliff, T.L. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J. Urol. 1995, 154, 407–413. [Google Scholar] [CrossRef]
- Irani, J.; Levillain, P.; Goujon, J.M.; Bon, D.; Doré, B.; Aubert, J. Inflammation in benign prostatic hyperplasia: Correlation with prostate specific antigen value. J. Urol. 1997, 157, 1301–1303. [Google Scholar] [CrossRef]
- Morote, J.; Lopez, M.; Encabo, G.; de Torres, I.M. Effect of inflammation and benign prostatic enlargement on total and percent free serum prostatic specific antigen. Eur. Urol. 2000, 37, 537–540. [Google Scholar] [CrossRef]
- Schaeffer, A.J.; Wu, S.C.; Tennenberg, A.M.; Kahn, J.B. Treatment of chronic bacterial prostatitis with levofloxacin and ciprofloxacin lowers serum prostate specific antigen. J. Urol. 2005, 174, 161–164. [Google Scholar] [CrossRef]
- Stevermer, J.J.; Easley, S.K. Treatment of prostatitis. Am. Fam. Physician 2000, 61, 3015–3026. [Google Scholar]
- Van den Bogert, C.; Dontje, B.H.; Kroon, A.M. The antitumour effect of doxycycline on a T-cell leukaemia in the rat. Leuk. Res. 1985, 9, 617–623. [Google Scholar] [CrossRef]
- Liu, J.; Kuszynski, C.A.; Baxter, B.T. Doxycycline induces Fas/Fas ligand-mediated apoptosis in Jurkat T lymphocytes. Biochem. Biophys. Res. Commun. 1999, 260, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Mouradis PX, E.; Colston, K.W.; Dalgleish, A.G. Doxycycline induces caspase dependent apoptosis in human pancreatic cancer cells. Int. J. Cancer 2007, 120, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fares, M.; Maguire, K.R.; Sidén, Å.; Potácová, Z. Cytotoxic Effects of Tetracycline Analogues (Doxycycline, Minocycline and COL-3) in Acute Myeloid Leukemia HL-60 Cells. PLoS ONE 2014, 9, e114457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijk, S.N.; Protasoni, M.; Elpidorou, M.; Kroon, A.M.; Taanman, J.W. Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: The effects of doxycycline and gemcitabine. Sci. Rep. 2020, 10, 4363. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Pyroptosis. Curr. Biol. CB 2016, 26, R568–R572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Zhou, Q.; Fan, C.; Zhao, H.; Wang, Y.; Qiu, X.; Yang, K.; Ji, Q. Doxycycline inhibits NAcht Leucine-rich repeat Protein 3 inflammasome activation and interleukin-1β production induced by Porphyromonas gingivalis-lipopolysaccharide and adenosine triphosphate in human gingival fibroblasts. Arch. Oral Biol. 2019, 107, 104514. [Google Scholar] [CrossRef]
- Apte, R.N.; Voronov, E. Immunotherapeutic approaches of IL-1 neutralization in the tumor microenvironment. J. Leukoc. Biol. 2017, 102, 293–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culig, Z.; Puhr, M. Interleukin-6 and prostate cancer: Current developments and unsolved questions. Mol. Cell Endocrinol. 2018, 462, 25–30. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [Green Version]
- Graven, S.N.; Estrada-O, S.; Lardy, H.A. Alkali metal cation release and respiratory inhibition induced by nigericin in rat liver mitochondria. Proc. Natl. Acad. Sci. USA 1966, 56, 654–658. [Google Scholar] [CrossRef] [Green Version]
- Laliberte, R.E.; Eggler, J.; Gabel, C.A. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J. Biol. Chem. 1999, 274, 36944–36951. [Google Scholar] [CrossRef] [Green Version]
- Lagarkova, M.A.; Shutova, M.V.; Bogomazova, A.N.; Vassina, E.M.; Glazov, E.A.; Zhang, P.; Rizvanov, A.A.; Chestkov, I.V.; Kiselev, S.L. Induction of pluripotency in human endothelial cells resets epigenetic profile on genome scale. Cell Cycle (Georgetown, Tex.) 2010, 9, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Alexander-Savino, C.V.; Hayden, M.S.; Richardson, C.; Zhao, J.; Poligone, B. Doxycycline is an NF-κB inhibitor that induces apoptotic cell death in malignant T-cells. Oncotarget 2016, 7, 75954–75967. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, T.; Mansour, A.; Spaczynski, R.Z.; Duleba, A.J. Effects of transforming growth factors-alpha and -beta on proliferation and apoptosis of rat theca-interstitial cells. J. Endocrinol. 2001, 170, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Goodsell, D.S. The molecular perspective: Epidermal growth factor. Oncol. 2003, 21, 702–703. [Google Scholar] [CrossRef]
- Kim, H.R.; Heo, Y.M.; Jeong, K.I.; Kim, Y.M.; Jang, H.L.; Lee, K.Y.; Yeo, C.Y.; Kim, S.H.; Lee, H.K.; Kim, S.R.; et al. FGF-2 inhibits TNF-α mediated apoptosis through upregulation of Bcl2-A1 and Bcl-xL in ATDC5 cells. BMB Rep. 2012, 45, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, I.S. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp. Mol. Med. 2016, 48, e242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, G.; Garanina, E.E.; Zhuravleva, M.N.; Hamza, S.; Rizvanov, A.A.; Khaiboullina, S.F. Rab GTPase Mediating Regulation of NALP3 in Colorectal Cancer. Molecules (Basel, Switzerland) 2020, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaadi, M.; Tezcan, G.; Garanina, E.E.; Hamza, S.; McIntyre, A.; Rizvanov, A.A.; Khaiboullina, S.F. Doxycycline Attenuates Cancer Cell Growth by Suppressing NLRP3-Mediated Inflammation. Pharmaceuticals 2021, 14, 852. https://doi.org/10.3390/ph14090852
Alsaadi M, Tezcan G, Garanina EE, Hamza S, McIntyre A, Rizvanov AA, Khaiboullina SF. Doxycycline Attenuates Cancer Cell Growth by Suppressing NLRP3-Mediated Inflammation. Pharmaceuticals. 2021; 14(9):852. https://doi.org/10.3390/ph14090852
Chicago/Turabian StyleAlsaadi, Mohammad, Gulcin Tezcan, Ekaterina E. Garanina, Shaimaa Hamza, Alan McIntyre, Albert A. Rizvanov, and Svetlana F. Khaiboullina. 2021. "Doxycycline Attenuates Cancer Cell Growth by Suppressing NLRP3-Mediated Inflammation" Pharmaceuticals 14, no. 9: 852. https://doi.org/10.3390/ph14090852
APA StyleAlsaadi, M., Tezcan, G., Garanina, E. E., Hamza, S., McIntyre, A., Rizvanov, A. A., & Khaiboullina, S. F. (2021). Doxycycline Attenuates Cancer Cell Growth by Suppressing NLRP3-Mediated Inflammation. Pharmaceuticals, 14(9), 852. https://doi.org/10.3390/ph14090852