Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications
Abstract
:1. Introduction
2. Results
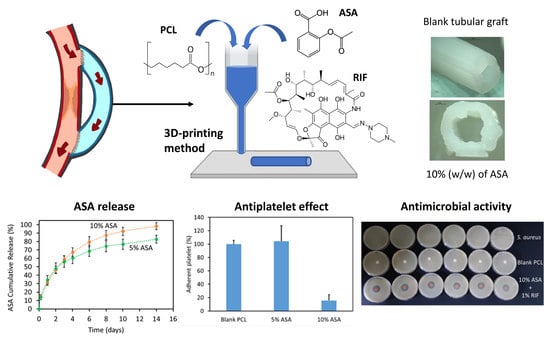
2.1. Three-Dimensional Printing of PCL-Based Materials Loaded with ASA
2.2. Characterisation of Tubular 3D-Printed Tubular Grafts
2.3. ASA Release Kinetics
2.4. Platelet Adhesion Study
2.5. Three-Dimensional Printing and Characterization of Grafts Containing RIF
2.6. Cell Proliferation Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Three-Dimensional Printed Tubular Grafts Design and Manufacture
4.3. Characterisation of the 3D-Printed Materials
4.4. ASA Release Kinetics
4.5. Platelet Adhesion Study
4.6. Cell Proliferation Study
4.7. Microbiological Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, N.K.; Domínguez-Robles, J.; Stewart, S.A.; Cornelius, V.A.; Anjani, Q.K.; Utomo, E.; García-Romero, I.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; et al. Fused deposition modelling for the development of drug loaded cardiovascular prosthesis. Int. J. Pharm. 2021, 595, 120243. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Sharkawi, T.; Cornhill, F.; Lafont, A.; Sabaria, P.; Vert, M. Intravascular bioresorbable polymeric stents: A potential alternative to current drug eluting metal stents. J. Pharm. Sci. 2007, 96, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, A.M.; Yang, Y.P. Tunable Elastomers with an Antithrombotic Component for Cardiovascular Applications. Adv. Healthc. Mater. 2018, 7, e1800222. [Google Scholar] [CrossRef] [PubMed]
- Roll, S.; Müller-Nordhorn, J.; Keil, T.; Scholz, H.; Eidt, D.; Greiner, W.; Willich, S.N. Dacron® vs. PTFE as bypass materials in peripheral vascular surgery-systematic review and meta-analysis. BMC Surg. 2008, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Dieval, F.; Khoffi, F.; Mir, R.; Chaouch, W.; Le Nouen, D.; Chakfe, N.; Durand, B. Long-Term Biostability of Pet Vascular Prostheses. Int. J. Polym. Sci. 2012, 2012, 646578. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Punnakitikashem, P.; Truong, D.; Menon, J.U.; Nguyen, K.T.; Hong, Y. Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts. Acta Biomater. 2014, 10, 4618–4628. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Shen, T.; Cornelius, V.A.; Corduas, F.; Mancuso, E.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; Larrañeta, E. Development of drug loaded cardiovascular prosthesis for thrombosis prevention using 3D printing. Mater. Sci. Eng. C 2021, 129, 112375. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Rossi, S.; Azghani, A.O.; Omri, A. Antimicrobial efficacy of a new antibiotic-loaded poly(hydroxybutyric-co-hydroxyvaleric acid) controlled release system. J. Antimicrob. Chemother. 2004, 54, 1013–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, L.K.; Saltzman, W.M. Polymeric implants for cancer chemotherapy. Adv. Drug Deliv. Rev. 1997, 26, 209–230. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Utomo, E.; Picco, C.J.; Corduas, F.; Mancuso, E.; Amir, M.N.; Bahar, M.A.; Sumarheni, S.; Donnelly, R.F.; et al. Poly(caprolactone)-based subcutaneous implant for sustained delivery of levothyroxine. Int. J. Pharm. 2021, 607, 121011. [Google Scholar] [CrossRef]
- Farmer, Z.-L.; Utomo, E.; Domínguez-Robles, J.; Mancinelli, C.; Mathew, E.; Larrañeta, E.; Lamprou, D.A. 3D printed estradiol-eluting urogynecological mesh implants: Influence of material and mesh geometry on their mechanical properties. Int. J. Pharm. 2021, 593, 120145. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Mancinelli, C.; Mancuso, E.; García-Romero, I.; Gilmore, B.F.; Casettari, L.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery. Pharmaceutics 2020, 12, 63. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.; Weisman, J.; Nicholson, C.; Jammalamadaka, U.; Tappa, K.; Wilson, C. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Mathew, E.; Domínguez-Robles, J.; Stewart, S.A.; Mancuso, E.; O’Donnell, K.; Larrañeta, E.; Lamprou, D.A. Fused Deposition Modeling as an Effective Tool for Anti-Infective Dialysis Catheter Fabrication. ACS Biomater. Sci. Eng. 2019, 5, 6300–6310. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Ercolani, E.; Galloni, P.; Santilli, F.; Baiguera, S.; Polizzi, L.; Bianco, A. Aspirin-loaded electrospun poly(ε-caprolactone) tubular scaffolds: Potential small-diameter vascular grafts for thrombosis prevention. J. Mater. Sci. Mater. Med. 2013, 24, 523–532. [Google Scholar] [CrossRef]
- Hou, D.; Huibregtse, B.; Dawkins, K.; Donnelly, J.; Roy, K.; Chen, J.P.; Akinapelli, A. Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents. Curr. Cardiol. Rev. 2017, 13, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Cerea, M.; Foppoli, A.; Maroni, A.; Moutaharrik, S.; Palugan, L.; Zema, L.; Gazzaniga, A. A Graphical Review on the Escalation of Fused Deposition Modeling (FDM) 3D Printing in the Pharmaceutical Field. J. Pharm. Sci. 2020, 109, 2943–2957. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arefin, A.; Khatri, N.; Kulkarni, N.; Egan, P. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Gonzalez, Z.; Utomo, E.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Poly(caprolactone)-Based Coatings on 3D-Printed Biodegradable Implants: A Novel Strategy to Prolong Delivery of Hydrophilic Drugs. Mol. Pharm. 2020, 17, 3487–3500. [Google Scholar] [CrossRef]
- Schrör, K. Aspirin and Platelets: The Antiplatelet Action of Aspirin and Its Role in Thrombosis Treatment and Prophylaxis. Semin. Thromb. Hemost. 1997, 23, 349–356. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Hirsh, J.; Spencer, F.A.; Baglin, T.P.; Weitz, J.I. Antiplatelet Drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e89S–e119S. [Google Scholar] [CrossRef] [Green Version]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Antithrombotic properties of aspirin and resistance to aspirin: Beyond strictly antiplatelet actions. Blood 2006, 109, 2285–2292. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.B.; Grove, E.; Neergaard-Petersen, S.; Würtz, M.; Hvas, A.-M.; Kristensen, S.D. Determinants of Reduced Antiplatelet Effect of Aspirin in Patients with Stable Coronary Artery Disease. PLoS ONE 2015, 10, e0126767. [Google Scholar] [CrossRef] [Green Version]
- Behan, M.W.H. Antiplatelet therapy in cardiovascular disease. Postgrad. Med. J. 2004, 80, 155–164. [Google Scholar] [CrossRef]
- Hall, J.D.; Rittgers, S.E.; Schmidt, S.P. Effect of Controlled Local Acetylsalicylic Acid Release on in vitro Platelet Adhesion to Vascular Grafts. J. Biomater. Appl. 1994, 8, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Akşit, E.; Kurt, T.; Büyük, B.; Ömer, C. Drug-eluting Vein Graft with Acetylsalicylic Acid-Ticagrelor-Unfractionated Heparin Complex Inhibits Early Graft Thrombosis. Balk. Med. J. 2020, 37, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jia, Y.; Yao, C.; Lu, Y. PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int. J. Pharm. 2014, 469, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, M.A.; Shahabadi, A.B.; Jarolmasjed, S.; Memar, M.Y.; Dizaj, S.M.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Zhen, X.-E.; Zong, M.; Gao, S.-N.; Cao, Y.-G.; Jiang, L.; Chen, S.-X.; Wang, K.; Sun, S.-Q.; Peng, H.-S.; Bai, Y.-H.; et al. Preparation and Characterization of a Novel Aspirin Derivative with Anti-Thrombotic and Gastric Mucosal Protection Properties. PLoS ONE 2014, 9, e98513. [Google Scholar] [CrossRef] [Green Version]
- Binev, I.; Stamboliyska, B.; Binev, Y. The infrared spectra and structure of acetylsalicylic acid (aspirin) and its oxyanion: An ab initio force field treatment. J. Mol. Struct. 1996, 378, 189–197. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Zajdel, P.; Pietrasik, E.; Barsova, Z.; Goryczka, T. Application of X-ray powder diffraction and differential scanning calorimetry for identification of counterfeit drugs. Mon. Chem. Chem. Mon. 2018, 149, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Gorniak, A.; Wojakowska, A.; Karolewicz, B.; Pluta, J. Phase diagram and dissolution studies of the fenofibrate–acetylsalicylic acid system. J. Therm. Anal. Calorim. 2010, 104, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Górniak, A.; Karolewicz, B.; Żurawska-Płaksej, E.; Pluta, J. Thermal, spectroscopic, and dissolution studies of the simvastatin–acetylsalicylic acid mixtures. J. Therm. Anal. Calorim. 2013, 111, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Martínez-Ohárriz, C.; Vélaz, I.; Zornoza, A.; Machín, R.; Isasi, J.R. In Vitro Release from Reverse Poloxamine/α-Cyclodextrin Matrices: Modelling and Comparison of Dissolution Profiles. J. Pharm. Sci. 2014, 103, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslakci, N.N.; Ulusoy, S.; Uygun, E.; Çevikbaş, H.; Oksuz, L.; Can, H.K.; Oksuz, A.U. Ibuprofen and acetylsalicylic acid loaded electrospun PVP-dextran nanofiber mats for biomedical applications. Polym. Bull. 2017, 74, 3283–3299. [Google Scholar] [CrossRef]
- Shalla, A.; Bhat, M. Smart polymer composites in drug delivery. In Smart Polymer Nanocomposites; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 261–294. [Google Scholar]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly(butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Spadaccio, C.; Chello, M.; Trombetta, M.; Rainer, A.; Toyoda, Y.; Genovese, J.A. Drug releasing systems in cardiovascular tissue engineering. J. Cell. Mol. Med. 2009, 13, 422–439. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, X.; Jiang, L.; Zhang, L.; Dong, Y.; Midgley, A.C.; Kong, D.; Wang, S. Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration. Acta Biomater. 2019, 97, 360–373. [Google Scholar] [CrossRef]
- Allen, B.; Sparks, R.; Welch, M.; Mason, N.; Mathias, C.; Clark, R. Reduction of platelet deposition on vascular grafts using an antiplatelet graft coating technique. J. Surg. Res. 1984, 36, 80–88. [Google Scholar] [CrossRef]
- Mathew, E.; Domínguez-Robles, J.; Larrañeta, E.; Lamprou, D.A. Fused Deposition Modelling as a Potential Tool for Antimicrobial Dialysis Catheters Manufacturing: New Trends vs. Conventional Approaches. Coatings 2019, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Caracciolo, P.C.; Rial-Hermida, M.I.; Montini-Ballarin, F.; Abraham, G.; Concheiro, A.; Alvarez-Lorenzo, C. Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts. Mater. Sci. Eng. C 2017, 75, 1115–1127. [Google Scholar] [CrossRef]
- Guan, G.; Yu, C.; Xing, M.; Wu, Y.; Hu, X.; Wang, H.; Wang, L. Hydrogel Small-Diameter Vascular Graft Reinforced with a Braided Fiber Strut with Improved Mechanical Properties. Polymers 2019, 11, 810. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Policianova, O.; Brus, J.; Hruby, M.; Urbanova, M.; Zhigunov, A.; Kredatusova, J.; Kobera, L. Structural Diversity of Solid Dispersions of Acetylsalicylic Acid As Seen by Solid-State NMR. Mol. Pharm. 2014, 11, 516–530. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, Y.; Fu, J.; Wan, Y.; Yang, R.; Gao, W.; Wang, H. Evaluation of drug release property and blood compatibility of aspirin-loaded electrospun PLA/RSF composite nanofibers. Iran. Polym. J. 2013, 22, 729–737. [Google Scholar] [CrossRef]
- Linneweber, J.; Dohmen, P.M.; Kerzscher, U.; Affeld, K.; Nosé, Y.; Konertz, W. The Effect of Surface Roughness on Activation of the Coagulation System and Platelet Adhesion in Rotary Blood Pumps. Artif. Organs 2007, 31, 345–351. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Schubert, P.; Toosi, S.F.; Chen, Z.; Culibrk, B.; Ramirez-Arcos, S.; Devine, D.V.; Brooks, D.E. Effect of texture of platelet bags on bacterial and platelet adhesion. Transfusion 2016, 56, 2808–2818. [Google Scholar] [CrossRef]
- Movahedi, M.; Salehi, A.O.M.; Moezi, D.; Yarahmadian, R. In vitro and in vivo study of aspirin loaded, electrospun polycaprolactone–maltodextrin membrane for enhanced skin tissue regeneration. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–11. [Google Scholar] [CrossRef]
- Aslani, S.; Kabiri, M.; Kehtari, M.; Hanaee-Ahvaz, H. Vascular tissue engineering: Fabrication and characterization of acetylsalicylic acid-loaded electrospun scaffolds coated with amniotic membrane lysate. J. Cell. Physiol. 2019, 234, 16080–16096. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Lee, M.-K.; Park, C.; Jung, H.-D.; Kim, H.-E.; Jang, T.-S. Fabrication of strong, bioactive vascular grafts with PCL/collagen and PCL/silica bilayers for small-diameter vascular applications. Mater. Des. 2019, 181, 108079. [Google Scholar] [CrossRef]
- Liu, S.-J.; Lee, C.-H.; Lin, Y.-H.; Tai, C.-D.; Hsieh, M.-J.; Chang, S.-H.; Chu, Y.; Hsu, M.-Y.; Chang, H.; Chang, G.-J.; et al. Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery. Int. J. Nanomed. 2014, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinbold, J.; Hierlemann, T.; Urich, L.; Uhde, A.-K.; Müller, I.; Weindl, T.; Vogel, U.; Schlensak, C.; Wendel, H.-P.; Krajewski, S. Biodegradable rifampicin-releasing coating of surgical meshes for the prevention of bacterial infections. Drug Des. Dev. Ther. 2017, 11, 2753–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mândru, M.; Ciobanu, C.; Vlad, S.; Butnaru, M.; Lebrun, L.; Popa, M. Characteristics of polyurethane-based sustained release membranes for drug delivery. Open Chem. 2013, 11, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Elens, M.; Dusoruth, M.; Astarci, P.; Mastrobuoni, S.; Bosiers, M.J.; Nardella, J.; Lacroix, V.; Possoz, J.; Verhelst, R. Management and Outcome of Prosthetic Vascular Graft Infections: A Single Center Experience. Vasc. Endovasc. Surg. 2018, 52, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sigot-Luizard, M.F.; Warocquier-Clerout, R. In Vitro Cytocompatibility Tests. In Test Procedures for the Blood Compatibility of Biomaterials; Springer: Dordrecht, The Netherlands, 1993; pp. 569–594. [Google Scholar]
- Zhan, J.; Feng, F.; Xu, M.; Yao, L.; Ge, M. Progress in Chemo–Mechanical Interactions between Nanoparticles and Cells. Acta Phys. Chim. Sin. 2020, 36, 1905076. [Google Scholar] [CrossRef]
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Pizzoferrato, A.; Ciapetti, G.; Stea, S.; Cenni, E.; Arciola, C.R.; Granchi, D.; Lucia. Cell culture methods for testing Biocompatibility. Clin. Mater. 1994, 15, 173–190. [Google Scholar] [CrossRef]
- International Organization for Standardisation. ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 18 August 2021).
- Dujić, T.; Causević, A.; Malenica, M. The Effects of Different Concentrations of Acetylsalicylic Acid on Proliferation and Viability of Lymphocytes in Cell Culture. Bosn. J. Basic Med. Sci. 2008, 8, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, T.; Kawazoe, N.; Tateishi, T.; Chen, G. In vitro evaluation of biodegradation of poly(lactic-co-glycolic acid) sponges. Biomaterials 2008, 29, 3438–3443. [Google Scholar] [CrossRef]
- Dey, J.; Xu, H.; Shen, J.; Thevenot, P.; Gondi, S.R.; Nguyen, K.T.; Sumerlin, B.; Tang, L.; Yang, J. Development of biodegradable crosslinked urethane-doped polyester elastomers. Biomaterials 2008, 29, 4637–4649. [Google Scholar] [CrossRef] [Green Version]








| ASA (%) | Korsmeyer–Peppas | Higuchi | Zero-Order | ||||
|---|---|---|---|---|---|---|---|
| KKP | n | r2 | KH | r2 | KZO | r2 | |
| 5 | 0.34 ± 0.01 | 0.45 ± 0.01 | 0.9930 | 0.32 ± 0.01 | 0.9899 | 0.14 ± 0.03 | 0.8820 |
| 10 | 0.32 ± 0.01 | 0.54 ± 0.02 | 0.9920 | 0.33 ± 0.01 | 0.9974 | 0.18 ± 0.02 | 0.9483 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. https://doi.org/10.3390/ph14090921
Domínguez-Robles J, Diaz-Gomez L, Utomo E, Shen T, Picco CJ, Alvarez-Lorenzo C, Concheiro A, Donnelly RF, Larrañeta E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals. 2021; 14(9):921. https://doi.org/10.3390/ph14090921
Chicago/Turabian StyleDomínguez-Robles, Juan, Luis Diaz-Gomez, Emilia Utomo, Tingjun Shen, Camila J. Picco, Carmen Alvarez-Lorenzo, Angel Concheiro, Ryan F. Donnelly, and Eneko Larrañeta. 2021. "Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications" Pharmaceuticals 14, no. 9: 921. https://doi.org/10.3390/ph14090921
APA StyleDomínguez-Robles, J., Diaz-Gomez, L., Utomo, E., Shen, T., Picco, C. J., Alvarez-Lorenzo, C., Concheiro, A., Donnelly, R. F., & Larrañeta, E. (2021). Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals, 14(9), 921. https://doi.org/10.3390/ph14090921