Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review
Abstract
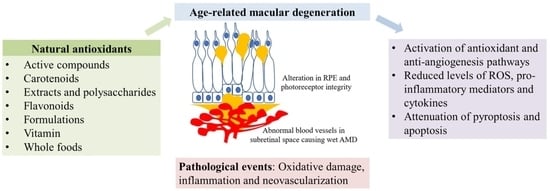
:1. Introduction
1.1. Pathogenesis of AMD
1.1.1. Features of Choroidal Neovascularization
1.1.2. Features of Geographic Atrophy
1.2. Standard Treatment Options for Wet AMD
1.3. Adverse Events following Standard Treatment for Wet AMD
1.4. Role of Natural Antioxidants for AMD
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Analysis
3. Results
Study Selection
4. Discussion
4.1. Active Compounds
4.1.1. Alkaloids
4.1.2. Curcumin
4.1.3. Ginsenoside
4.1.4. Other Active Compounds
4.2. Carotenoids
4.3. Extracts and Polysaccharides
4.4. Flavonoids
4.5. Formulations
4.6. Vitamins
4.7. Whole Foods
4.7.1. Saffron
4.7.2. Other Whole Foods
5. Limitations and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection For 2020 and 2040: A Systematic Review and Meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wu, J.; Yu, X.; Tang, Y.; Tang, X.; Shentu, X. Regional Differences in the Global Burden of Age-Related Macular Degeneration. BMC Public Health 2020, 20, 410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Jonas, J.B.; Yang, H.; Ma, Y.; Li, J. Prevalence of Age-Related Maculopathy in the Adult Population in China: The Beijing Eye Study. Am. J. Ophthalmol. 2006, 142, 788-793.e1. [Google Scholar] [CrossRef] [PubMed]
- Joachim, N.; Mitchell, P.; Younan, C.; Burlutsky, G.; Cheng, C.Y.; Cheung, C.M.G.; Zheng, Y.; Moffitt, M.; Wong, T.Y.; Wang, J.J. Ethnic Variation in Early Age-Related Macular Degeneration Lesions between White Australians and Singaporean Asians. Investig. Opthalmology Vis. Sci. 2014, 55, 4421–4429. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Li, X.; Cheng, C.Y.; Zheng, Y.; Mitchell, P.; Wang, J.J.; Wong, T.Y. Prevalence, Racial Variations, and Risk Factors of Age-Related Macular Degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology 2014, 121, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, H.; Karimi, S.; Ahmadieh, H.; Azarmina, M.; Abrishami, M.; Ahoor, H.; Alizadeh, Y.; Behboudi, H.; Daftarian, N.; Dehghan, M.; et al. Intravitreal Injection of Anti-Vascular Endothelial Growth Factor Agents for Ocular Vascular Diseases: Clinical Practice Guideline. J. Ophthalmic Vis. Res. 2018, 13, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Velilla, S.; Garcia-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Duran, M.D.; Gómez-Ulla, F.; Arevalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and Age-Related Macular Degeneration: Review and Update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence From a Review of the Molecular Mechanisms and Animal Models. Oxidative Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships Between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch, D.G.; Liang, F.Q. Age-Related Macular Degeneration: A Target for Nanotechnology Derived Medicines. Int. J. Nanomed. 2007, 2, 65–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolf, M.; Clark, M.E.; Chimento, M.F.; Li, C.M.; Medeiros, N.E.; Curcio, C.A. Prevalence and Morphology of Druse Types in the Macula and Periphery of Eyes with Age-Related Maculopathy. Investig. Opthalmology Vis. Sci. 2008, 49, 1200–1209. [Google Scholar] [CrossRef]
- Khetan, V.; Palkar, A.H. Polypoidal Choroidal Vasculopathy: An Update on Current Management and Review of Literature. Taiwan J. Ophthalmol. 2019, 9, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Kim, I.K. Clinical Characteristics and Current Treatment of Age-Related Macular Degeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a017178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaszubski, P.; Ben Ami, T.; Saade, C.; Smith, R.T. Geographic Atrophy and Choroidal Neovascularization in the Same Eye: A Review. Ophthalmic Res. 2016, 55, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.J.; Cheng, C.K.; Yeung, L.; Yang, C.H.; Chen, S.J.; Taiwan PCV Consensus Group. Management of Polypoidal Choroidal Vasculopathy: Experts Consensus in Taiwan. J. Formos. Med. Assoc. 2020, 119, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Bergh, H.V.D. Photodynamic Therapy of Age-Related Macular Degeneration: History and Principles. Semin. Ophthalmol. 2001, 16, 181–200. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Verteporfin. Drugs Aging 2000, 16, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Told, R.; Sacu, S.; Bandello, F.; Moisseiev, E.; Loewenstein, A.; Schmidt-Erfurth, U.; Euretina Board. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica 2018, 239, 181–193. [Google Scholar] [CrossRef]
- Brown, D.M.; Michels, M.; Kaiser, P.; Heier, J.S.; Sy, J.P.; Ianchulev, T. Ranibizumab Versus Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration: Two-Year Results of the ANCHOR Study. Ophthalmology 2009, 116, 57-65.e5. [Google Scholar] [CrossRef]
- Chen, Y.; Sharma, T.; Li, X.; Song, Y.; Chang, Q.; Lin, R.; Egger, A.; Foo, A.; Gekkieva, M.; Lai, T.Y.Y. Ranibizumab Versus Verteporfin Photodynamic Therapy in Asian Patients with Myopic Choroidal Neovascularization: BRILLIANCE, A 12-Month, Randomized, Double-Masked Study. Retina 2019, 39, 1985–1994. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, T.; Li, X.; Zhao, M.; Xu, X. Polypoidal Choroidal Vasculopathy Treatment Options: A Meta-Analysis. Eur. J. Clin. Investig. 2017, 48, e12840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.H.; Lai, T.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F.; et al. Comparison of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Antoszyk, A.N.; Tuomi, L.; Chung, C.Y.; Singh, A.; FOCUS Study Group. Ranibizumab Combined with Verteporfin Photodynamic Therapy in Neovascular Age-Related Macular Degeneration (FOCUS): Year 2 Results. Am. J. Ophthalmol. 2008, 145, 862-874.e3. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Jorge, R.; Calucci, D.; Melo, L.A.S.; Cardillo, J.A.; Scott, I.U. Intravitreal Bevacizumab (Avastin) in Combination with Verteporfin Photodynamic Therapy for Choroidal Neovascularization Associated with Age-Related Macular Degeneration (IBeVe Study). Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Registry of Visudyne AMD Therapy Writing Committee; Boyer, D.S.; Garcia, R.; Hao, Y.; Hughes, M.S.; Jabbour, N.M.; Kaiser, P.K.; Mieler, W.; Slakter, J.S.; et al. Verteporfin Photodynamic Therapy Combined with Intravitreal Bevacizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2009, 116, 747-755.e1. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, J.; Liu, Q.; Ren, C.; Cai, W.; Liang, X.; Wen, J.; Yu, J. Combination of Bevacizumab and Photodynamic Therapy Vs. Bevacizumab Monotherapy for the Treatment of Wet Age-Related Macular Degeneration: A Meta-Analysis of Randomized Controlled Trials. Exp. Ther. Med. 2018, 16, 1187–1194. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Maruyama-Inoue, M.; Kitajima, Y.; Sato, S.; Inoue, T.; Yamane, S.; Kadonosono, K. Comparison of One-Year Results of Photodynamic Therapy Combined with Ranibizumab or Aflibercept for Treating Polypoidal Choroidal Vasculopathy. PLoS ONE 2020, 15, e0235213. [Google Scholar] [CrossRef]
- Holekamp, N.; Wykoff, C.C.; Schmitz-Valckenberg, S.; Monés, J.; Souied, E.H.; Lin, H.; Rabena, M.D.; Cantrell, R.A.; Henry, E.C.; Tang, F.; et al. Natural History of Geographic Atrophy Secondary to Age-Related Macular Degeneration: Results from the Prospective Proxima A and B Clinical Trials. Ophthalmology 2020, 127, 769–783. [Google Scholar] [CrossRef] [Green Version]
- Al-Zamil, W.M.; Yassin, S.A. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [Green Version]
- Schnurrbusch, U.E.K.; Jochmann, C.; Einbock, W.; Wolf, S. Complications After Photodynamic Therapy. Arch. Ophthalmol. 2005, 123, 1347–1350. [Google Scholar] [CrossRef] [Green Version]
- Tzekov, R.; Lin, T.; Zhang, K.M.; Jackson, B.; Oyejide, A.; Orilla, W.; Kulkarni, A.D.; Kuppermann, B.D.; Wheeler, L.; Burke, J. Ocular Changes After Photodynamic Therapy. Investig. Opthalmol. Vis. Sci. 2006, 47, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Reinke, M.H.; Canakis, C.; Husain, D.; Michaud, N.; Flotte, T.J.; Gragoudas, E.S.; Miller, J.W. Verteporfin Photodynamic Therapy Retreatment of Normal Retina and Choroid in the Cynomolgus Monkey. Ophthalmology 1999, 106, 1915–1923. [Google Scholar] [CrossRef]
- Husain, D.; Miller, J.W.; Michaud, N.; Connolly, E.; Flotte, T.J.; Gragoudas, E.S. Intravenous Infusion of Liposomal Benzoporphyrin Derivative for Photodynamic Therapy of Experimental Choroidal Neovascularization. Arch. Ophthalmol. 1996, 114, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration with Verteporfin: One-Year Results of 2 Randomized Clinical Trials—TAP Report. Arch. Ophthalmol. 1999, 117, 1329–1345. [Google Scholar] [CrossRef] [Green Version]
- Bressler, N.M.; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic Therapy of Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration with Verteporfin: Two-Year Results of 2 Randomized Clinical Trials—TAP Report 2. Arch. Ophthalmol. 2001, 119, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.K. Photodynamic Therapy: Current Role in the Treatment of Chorioretinal Conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falavarjani, K.G.; Modarres, M.; Hashemi, M.; Parvaresh, M.M.; Naseripour, M.; Zare-Moghaddam, A.; Nekoozadeh, S. Incidence of Acute Endophthalmitis After Intravitreal Bevacizumab Injection in a Single Clinical Center. Retina 2013, 33, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, F.; Hammer, T.; Grueb, M.; Mueller, B.; Berk, H.; Gamulescu, M.A.; Voegeler, J.; Wachtlin, J.; Ocean Study Group. Reporting of Safety Events During Anti-VEGF Treatment: Pharmacovigilance in a Noninterventional Trial. J. Ophthalmol. 2020, 2020, 8652370. [Google Scholar] [CrossRef]
- Ueta, T.; Yanagi, Y.; Tamaki, Y.; Yamaguchi, T. Cerebrovascular Accidents in Ranibizumab. Ophthalmology 2009, 116, 362.e1. [Google Scholar] [CrossRef]
- Tolentino, M. Systemic and Ocular Safety of Intravitreal Anti-VEGF Therapies for Ocular Neovascular Disease. Surv. Ophthalmol. 2011, 56, 95–113. [Google Scholar] [CrossRef]
- Singer, M.A.; Awh, C.C.; Sadda, S.; Freeman, W.R.; Antoszyk, A.N.; Wong, P.; Tuomi, L. HORIZON: An Open-Label Extension Trial of Ranibizumab for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Ophthalmology 2012, 119, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Bracha, P.; Moore, N.A.; Ciulla, T.A. Induced Pluripotent Stem Cell-Based Therapy for Age-Related Macular Degeneration. Expert Opin. Biol. Ther. 2017, 17, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.N.; Hariprasad, S.M.; Sheth, V.S. An Overview of the Fovista and Rinucumab Trials and the Fate of Anti-PDGF Medications. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Maftouhi, M.Q.-E.; et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: A Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Malek, G.; Busik, J.; Grant, M.B.; Choudhary, M. Models of Retinal Diseases and Their Applicability in Drug Discovery. Expert Opin. Drug Discov. 2017, 13, 359–377. [Google Scholar] [CrossRef]
- Gomi, F.; Sawa, M.; Sakaguchi, H.; Tsujikawa, M.; Oshima, Y.; Kamei, M.; Tano, Y. Efficacy of Intravitreal Bevacizumab for Polypoidal Choroidal Vasculopathy. Br. J. Ophthalmol. 2007, 92, 70–73. [Google Scholar] [CrossRef]
- Lai, T.Y.Y.; Chan, W.M.; Liu, D.T.L.; Luk, F.O.J.; Lam, D.S.C. Intravitreal Bevacizumab (Avastin) With or Without Photodynamic Therapy for the Treatment of Polypoidal Choroidal Vasculopathy. Br. J. Ophthalmol. 2008, 92, 661–666. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Ooto, S.; Yamashiro, K.; Tamura, H.; Otani, A.; Yoshimura, N. Treatment of Polypoidal Choroidal Vasculopathy by Intravitreal Injection of Bevacizumab. Jpn. J. Ophthalmol. 2010, 54, 310–319. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative Stress, Innate Immunity, and Age-Related Macular Degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated With Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxidative Med. Cell. Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue Light-Induced Inflammatory Marker Expression in the Retinal Pigment Epithelium-Choroid of Mice and the Protective Effect of a Yellow Intraocular Lens Material In Vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein Acts Via Multiple Antioxidant Pathways in the Photo-Stressed Retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective Effect of Bilberry Extract in a Murine Model of Photo-Stressed Retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional Autophagy in RPE, A Contributing Factor in Age-Related Macular Degeneration. Cell Death Dis. 2018, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Mechanisms for Countering Oxidative Stress and Damage in Retinal Pigment Epithelium. Int. Rev. Cell Mol. Biol. 2012, 298, 135–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, M.; Milliner, C.; Bell, B.A.; Bonilha, V.L. Oxidative Stress in the Retina and Retinal Pigment Epithelium (RPE): Role of Aging, and DJ-1. Redox Biol. 2020, 37, 101623. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response Against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective Effects of Hericium erinaceus (Bull.: Fr.) Pers. Against High-Dose Corticosterone-Induced Oxidative Stress in PC-12 Cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Lew, S.Y.; Yow, Y.Y.; Lim, L.W.; Wong, K.H. Antioxidant-Mediated Protective Role of Hericium erinaceus (Bull.: Fr.) Pers. Against Oxidative Damage in Fibroblasts From Friedreich’s Ataxia Patient. Food Sci. Technol. 2020, 40, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Subermaniam, K.; Yow, Y.Y.; Lim, S.H.; Koh, O.H.; Wong, K.H. Malaysian Macroalga Padina australis Hauck Attenuates High Dose Corticosterone-Mediated Oxidative Damage in PC12 Cells Mimicking the Effects of Depression. Saudi J. Biol. Sci. 2020, 27, 1435–1445. [Google Scholar] [CrossRef]
- Carneiro, Â.; Andrade, J.P. Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 6469138. [Google Scholar] [CrossRef]
- Tu, G.; Zhang, Y.F.; Wei, W.; Li, L.; Zhang, Y.; Yang, J.; Xing, Y. Allicin Attenuates H2O2-Induced Cytotoxicity in Retinal Pigmented Epithelial Cells by Regulating the Levels of Reactive Oxygen Species. Mol. Med. Rep. 2016, 13, 2320–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Chaudhary, S.C.; Zhao, X.; Gaur, U.; Fang, J.; Yan, F.; Zheng, W. Artemisinin Protects Human Retinal Pigmented Epithelial Cells Against Hydrogen Peroxide-Induced Oxidative Damage by Enhancing the Activation of AMP-Active Protein Kinase. Int. J. Biol. Sci. 2019, 15, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shang, A.; Wang, W.; Yang, J. Astragaloside Suppresses Tumor Necrosis Factor Receptor-Associated Factor 5 Signaling Pathway and Alleviates Neurodegenerative Changes in Retinal Pigment Epithelial Cells Induced by Isoflurane. J. Cell. Biochem. 2019, 120, 1028–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Gaur, U.; Chong, C.M.; Lin, S.; Fang, J.; Zeng, Z.; Wang, H.; Zheng, W. Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage Through Activation of AMPK. Int. J. Mol. Sci. 2018, 19, 1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Jiang, Y.; Xing, X.; Lin, R.; Li, Q.; Zhou, W.; Qiu, W.; Zheng, W. Protective Mechanism of Berberine on Human Retinal Pigment Epithelial Cells Against Apoptosis Induced by Hydrogen Peroxide Via the Stimulation of Autophagy. Oxidative Med. Cell. Longev. 2021, 2021, 7654143. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Song, J.; Wang, C.; Li, Y.; Dunaief, J.L. Berberine Protects Against Light-Induced Photoreceptor Degeneration in the Mouse Retina. Exp. Eye Res. 2015, 145, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, T.; McKercher, S.R.; Kosaka, K.; Seki, M.; Wheeler, L.; Viswanath, V.; Chun, T.; Joshi, R.; Valencia, M.; Sasaki, S.; et al. Protective Effect of Carnosic Acid, a Pro-Electrophilic Compound, in Models of Oxidative Stress and Light-Induced Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2012, 53, 7847–7854. [Google Scholar] [CrossRef] [Green Version]
- Paimela, T.; Hyttinen, J.M.; Viiri, J.; Ryhänen, T.; Karjalainen, R.O.; Salminen, A.; Kaarniranta, K. Celastrol Regulates Innate Immunity Response Via NF-κB and Hsp70 in Human Retinal Pigment Epithelial Cells. Pharmacol. Res. 2011, 64, 501–508. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, W.; Wu, Y.; Meng, Y.F.; Wang, J.Y.; Xu, M.; Tao, J.J. Effect of Curcumin on Aging Retinal Pigment Epithelial Cells. Drug Des. Dev. Ther. 2015, 9, 5337–5344. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Chiu, H.F.; Han, Y.C.; Chen, I.H.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Photoprotective Effects of Cranberry Juice and Its Various Fractions Against Blue Light-Induced Impairment in Human Retinal Pigment Epithelial Cells. Pharm. Biol. 2016, 55, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.I.; Lee, E.H.; Kim, S.R.; Jang, Y.P. Anti-Apoptotic Effects of Curcuma longa L. Extract and Its Curcuminoids Against Blue Light-Induced Cytotoxicity in A2E-Laden Human Retinal Pigment Epithelial Cells. J. Pharm. Pharmacol. 2017, 69, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Sharif, U.; Na Bhuket, P.R.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate Against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef] [Green Version]
- Jitsanong, T.; Khanobdee, K.; Piyachaturawat, P.; Wongprasert, K. Diarylheptanoid 7-(3,4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene From Curcuma comosa Roxb. Protects Retinal Pigment Epithelial Cells Against Oxidative Stress-Induced Cell Death. Toxicol. Vitr. 2011, 25, 167–176. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Hong, S.H.; Kim, J.H.; Park, S.K.; Jeong, J.W.; Kim, G.Y.; Hyun, J.W.; Yun, S.J.; Kim, B.W.; et al. Protective Effect of Diphlorethohydroxycarmalol Against Oxidative Stress-Induced DNA Damage and Apoptosis in Retinal Pigment Epithelial Cells. Cutan. Ocul. Toxicol. 2019, 38, 298–308. [Google Scholar] [CrossRef]
- Cheng, L.B.; Chen, C.M.; Zhong, H.; Zhu, L.J. Squamosamide Derivative FLZ Protects Retinal Pigment Epithelium Cells From Oxidative Stress Through Activation of Epidermal Growth Factor Receptor (EGFR)-AKT Signaling. Int. J. Mol. Sci. 2014, 15, 18762–18775. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.T.; Liang, Z.Y.; Chen, S. Squamosamide Derivative FLZ Inhibits TNF-α-Induced ICAM-1 Expression Via Down-Regulation of the NF-κB Signaling Pathway in ARPE-19 Cells. Int. J. Clin. Exp. Pathol. 2015, 8, 9126–9132. [Google Scholar]
- Betts, B.S.; Parvathaneni, K.; Yendluri, B.B.; Grigsby, J.; Tsin, A.T.C. Ginsenoside-Rb1 Induces ARPE-19 Proliferation and Reduces VEGF Release. ISRN Ophthalmol. 2011, 2011, 184295. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Hussain, A.A.; Seok, J.H.; Kim, S.H.; Marshall, J. Modulating the Transport Characteristics of Bruch’s Membrane With Steroidal Glycosides and Its Relevance To Age-Related Macular Degeneration (AMD). Investig. Opthalmol. Vis. Sci. 2015, 56, 8403–8418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Wei, D.; Liu, H.; Zhu, C.; Lu, Y.; Ke, Z.; Jiang, S.; Huang, J. Glycyrrhizin Protects Against Sodium Iodate-Induced RPE and Retinal Injury Though Activation of AKT and Nrf2/HO-1 Pathway. J. Cell. Mol. Med. 2019, 23, 3495–3504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millan-Linares, M.C.; Toscano, R.; Lemus-Conejo, A.; Martin, M.E.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S. GPETAFLR, A Biopeptide From Lupinus angustifolius L., Protects Against Oxidative and Inflammatory Damage in Retinal Pigment Epithelium Cells. J. Food Biochem. 2019, 43, e12995. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Zeng, Z.; Graham, A.; Shu, X. Gypenosides Mediate Cholesterol Efflux and Suppress Oxidized LDL Induced Inflammation in Retinal Pigment Epithelium Cells. Exp. Eye Res. 2020, 191, 107931. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gu, S.; Zhang, Y.; Zhang, J. Kinsenoside Ameliorates Oxidative Stress-Induced RPE Cell Apoptosis and Inhibits Angiogenesis Via Erk/p38/NF-κB/VEGF Signaling. Front. Pharmacol. 2018, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; You, L.; Ni, B.; Sai, N.; Wang, W.; Sun, M.; Xu, R.; Yao, Y.; Zhang, Z.; Qu, C.; et al. Phillyrin Mitigates Apoptosis and Oxidative Stress in Hydrogen Peroxide-Treated RPE Cells Through Activation of the Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2684672. [Google Scholar] [CrossRef]
- Vieira, L.C.; Moreira, C.P.D.S.; Castro, B.F.M.; Cotta, O.A.L.; Silva, L.M.; Fulgêncio, G.D.O.; Silva-Cunha, A.; Fialho, S.L. Rosmarinic Acid Intravitreal Implants: A New Therapeutic Approach For Ocular Neovascularization. Planta Medica 2020, 86, 1286–1297. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Z.; Han, J.; Wei, G.; Yi, B.; Li, Z. Rhizoma paridis Total Saponins Alleviate H2O2-Induced Oxidative Stress Injury By Upregulating the Nrf2 Pathway. Mol. Med. Rep. 2019, 21, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic Potential of Curcumin in Eye Diseases. Central Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Kita, T.; Imai, S.; Sawada, H.; Kumagai, H.; Seto, H. The Biosynthetic Pathway of Curcuminoid in Turmeric (Curcuma longa) As Revealed By 13C-Labeled Precursors. Biosci. Biotechnol. Biochem. 2008, 72, 1789–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.C.; Chang, W.C.; Hung, K.H.; Yang, D.M.; Cheng, Y.H.; Liao, Y.W.; Woung, L.C.; Tsai, C.Y.; Hsu, C.C.; Lin, T.C.; et al. The Generation of Induced Pluripotent Stem Cells for Macular Degeneration as a Drug Screening Platform: Identification of Curcumin as a Protective Agent for Retinal Pigment Epithelial Cells Against Oxidative Stress. Front. Aging Neurosci. 2014, 6, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the Retina: The Best Is Yet To Come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef] [Green Version]
- Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, E.; Sahin, N.; Ozercan, I.H.; Namjoshi, T.; Srivastava, V.; Morde, A.; Rai, D.; et al. Different Doses of β-Cryptoxanthin May Secure the Retina From Photooxidative Injury Resulted From Common LED Sources. Oxidative Med. Cell. Longev. 2021, 2021, 6672525. [Google Scholar] [CrossRef]
- Karimi, P.; Gheisari, A.; Gasparini, S.J.; Baharvand, H.; Shekari, F.; Satarian, L.; Ader, M. Crocetin Prevents RPE Cells From Oxidative Stress Through Protection of Cellular Metabolic Function and Activation of ERK1/2. Int. J. Mol. Sci. 2020, 21, 2949. [Google Scholar] [CrossRef] [Green Version]
- Silván, J.M.; Reguero, M.; de Pascual-Teresa, S. A Protective Effect of Anthocyanins and Xanthophylls on UVB-Induced Damage in Retinal Pigment Epithelial Cells. Food Funct. 2016, 7, 1067–1076. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Investig. Opthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akuffo, K.; Beatty, S.; Peto, T.; Stack, J.; Stringham, J.; Kelly, D.; Leung, I.; Corcoran, L.; Nolan, J.M. The Impact of Supplemental Antioxidants on Visual Function in Nonadvanced Age-Related Macular Degeneration: A Head-To-Head Randomized Clinical Trial. Investig. Opthalmol. Vis. Sci. 2017, 58, 5347–5360. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-Up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Eye Disease Case Control Study Group. Antioxidant Status and Neovascular Age-Related Macular Degeneration. Arch. Ophthalmol. 1993, 111, 104–109. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, X.; Johnson, E.J.; Lim, A.; Sun, E.; Yu, J.; Zhang, Y.; Liu, X.; Snellingen, T.; Shang, F.; et al. Serum Carotenoids and Risk of Age-Related Macular Degeneration in a Chinese Population Sample. Investig. Opthalmol. Vis. Sci. 2011, 52, 4338–4344. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.C.; Rosen, R.B.; Farah, M. Macular Pigment in Retinal Health and Disease. Int. J. Retin. Vitr. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective Study of Lutein/Zeaxanthin Intake and Risk of Age-Related Macular Degeneration. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive Effect of Arctium lappa L. Leaves on Retinal Damage Against A2E-Induced ARPE-19 Cells and Mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins Via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Kuse, Y.; Tsuruma, K.; Kobayashi, S.; Shimazawa, M.; Hara, H. Protective Effects of Bilberry and Lingonberry Extracts Against Blue Light-Emitting Diode Light-Induced Retinal Photoreceptor Cell Damage In Vitro. BMC Complement. Altern. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [Green Version]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Fernández-Angulo, D.; Gil-Salido, A.A.; Lara-Espinoza, C.L.; Rubio-Pino, J.L. Retino-Protective Effect of Bucida buceras Against Oxidative Stress Induced By H2O2 in Human Retinal Pigment Epithelial Cells Line. BMC Complement. Altern. Med. 2015, 15, 254. [Google Scholar] [CrossRef] [Green Version]
- Park, D.W.; Lee, Y.G.; Jeong, Y.J.; Jeon, H.; Kang, S.C. Preventive Effects Against Retinal Degeneration by Centella asiatica Extract (CA-HE50) and Asiaticoside Through Apoptosis Suppression by the Nrf2/HO-1 Signaling Pathway. Antioxidants 2021, 10, 613. [Google Scholar] [CrossRef]
- Rohwer, K.; Neupane, S.; Bittkau, K.S.; Pérez, M.G.; Dörschmann, P.; Roider, J.; Alban, S.; Klettner, A. Effects of Crude Fucus distichus Subspecies evanescens Fucoidan Extract on Retinal Pigment Epithelium Cells―Implications for Use in Age-Related Macular Degeneration. Mar. Drugs 2019, 17, 538. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Kang, S.W.; Ahn, H.R.; Song, Y.; Yang, S.J.; Jung, S.H. Leaves of Persimmon (Diospyros kaki Thunb.) Ameliorate N-Methyl-N-nitrosourea (MNU)-Induced Retinal Degeneration in Mice. J. Agric. Food Chem. 2015, 63, 7750–7759. [Google Scholar] [CrossRef]
- Nashine, S.; Kanodia, R.; Nesburn, A.B.; Soman, G.; Kuppermann, B.D.; Kenney, M.C. Nutraceutical Effects of Emblica officinalis in Age-Related Macular Degeneration. Aging 2019, 11, 1177–1188. [Google Scholar] [CrossRef]
- Dithmer, M.; Fuchs, S.; Shi, Y.; Schmidt, H.; Richert, E.; Roider, J.; Klettner, A. Fucoidan Reduces Secretion and Expression of Vascular Endothelial Growth Factor in The Retinal Pigment Epithelium and Reduces Angiogenesis In Vitro. PLoS ONE 2014, 9, e89150. [Google Scholar] [CrossRef]
- Dörschmann, P.; Bittkau, K.S.; Neupane, S.; Roider, J.; Alban, S.; Klettner, A. Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells. Mar. Drugs 2019, 17, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörschmann, P.; Kopplin, G.; Roider, J.; Klettner, A. Effects of Sulfated Fucans from Laminaria hyperborea Regarding VEGF Secretion, Cell Viability, and Oxidative Stress and Correlation with Molecular Weight. Mar. Drugs 2019, 17, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörschmann, P.; Mikkelsen, M.D.; Thi, T.N.; Roider, J.; Meyer, A.S.; Klettner, A. Effects of a Newly Developed Enzyme-Assisted Extraction Method on the Biological Activities of Fucoidans in Ocular Cells. Mar. Drugs 2020, 18, 282. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Therapeutic Effect of Garcinia cambogia Extract and Hydroxycitric Acid Inhibiting Hypoxia-Inducible Factor in a Murine Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 5049. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sun, T.; Jiang, Y.; Wu, L.; Cai, X.; Sun, X.; Sun, X. Photooxidative Damage in Retinal Pigment Epithelial Cells Via GRP78 and The Protective Role of Grape Skin Polyphenols. Food Chem. Toxicol. 2014, 74, 216–224. [Google Scholar] [CrossRef]
- Ibuki, M.; Shoda, C.; Miwa, Y.; Ishida, A.; Tsubota, K.; Kurihara, T. Lactoferrin Has a Therapeutic Effect via HIF Inhibition in a Murine Model of Choroidal Neovascularization. Front. Pharmacol. 2020, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, F.-C.; Hung, C.-T.; Cheng, K.-C.; Wu, C.-Y.; Chen, Y.-C.; Wu, Y.-J.; Liu, W.; Chiu, C.-C. Protective Effects of Lycium barbarum Extracts on UVB-Induced Damage in Human Retinal Pigment Epithelial Cells Accompanied by Attenuating ROS and DNA Damage. Oxidative Med. Cell. Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef]
- Liu, L.; Lao, W.; Ji, Q.-S.; Yang, Z.-H.; Yu, G.-C.; Zhong, J.-X. Lycium barbarum Polysaccharides Protected Human Retinal Pigment Epithelial Cells Against Oxidative Stress-Induced Apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar] [CrossRef]
- Yang, M.; So, K.-F.; Lo, A.C.Y.; Lam, W.C. The Effect of Lycium barbarum Polysaccharides on Pyroptosis-Associated Amyloid β1-40 Oligomers-Induced Adult Retinal Pigment Epithelium 19 Cell Damage. Int. J. Mol. Sci. 2020, 21, 4658. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bao, S.; Du, Y.; Jiang, Z.; Wuliji, A.; Ren, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Antioxidant Effects of Lycium barbarum Polysaccharides on Photoreceptor Degeneration in The Light-Exposed Mouse Retina. Biomed. Pharmacother. 2018, 103, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.C.; Jee, D.; Rho, C.-R.; Kang, S. Melissa officinalis L. Extracts Protect Human Retinal Pigment Epithelial Cells Against Oxidative Stress-Induced Apoptosis. Int. J. Med. Sci. 2016, 13, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, N.R.; Pyun, B.-J.; Jung, D.H.; Lee, I.S.; Kim, C.-S.; Kim, Y.S.; Kim, J.S. Pueraria lobata Extract Protects Hydrogen Peroxide-Induced Human Retinal Pigment Epithelial Cells Death and Membrane Permeability. Evid.-Based Complement. Altern. Med. 2019, 2019, 5710289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornebise, C.; Courtaut, F.; Taillandier-Coindard, M.; Valls-Fonayet, J.; Richard, T.; Monchaud, D.; Aires, V.; Delmas, D. Red Wine Extract Inhibits VEGF Secretion and Its Signaling Pathway in Retinal ARPE-19 Cells to Potentially Disrupt AMD. Molecules 2020, 25, 5564. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Darrow, R.M.; Rapp, C.M.; Smuts, J.; Armstrong, D.; Lang, J.C. Prevention of Retinal Light Damage by Zinc Oxide Combined with Rosemary Extract. Mol. Vis. 2013, 19, 1433–1445. [Google Scholar] [PubMed]
- Wang, J.; Wang, Y.; Li, Q. Synthesis of AuNPs Using Plant Polyphenols and Their Potential Treatment for Age-Related Macular Degeneration. J. Drug Deliv. Sci. Technol. 2020, 55, 101377. [Google Scholar] [CrossRef]
- Pham, T.N.M.; Shin, C.-Y.; Park, S.H.; Lee, T.H.; Ryu, H.Y.; Kim, S.-B.; Auh, K.; Jeong, K.W. Solanum melongena L. Extract Protects Retinal Pigment Epithelial Cells from Blue Light-Induced Phototoxicity in In Vitro and In Vivo Models. Nutrients 2021, 13, 359. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, W.; He, X.; Zhang, D.; He, W. Tribulus terrestris Ameliorates Oxidative Stress-Induced ARPE-19 Cell Injury through the PI3K/Akt-Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7962393. [Google Scholar] [CrossRef]
- Yoon, S.-M.; Lee, B.-L.; Guo, Y.-R.; Choung, S.-Y. Preventive Effect of Vaccinium uliginosum L. Extract and Its Fractions on Age-Related Macular Degeneration and Its Action Mechanisms. Arch. Pharmacal Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Lee, B.-L.; Kang, J.-H.; Kim, H.-M.; Jeong, S.-H.; Jang, D.-S.; Jang, Y.-P.; Choung, S.-Y. Polyphenol-enriched Vaccinium uliginosum L. fractions reduce retinal damage induced by blue light in A2E-laden ARPE19 cell cultures and mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-Y.; Wu, H.; Li, D.-J.; Song, J.-F.; Xiao, Y.-D.; Liu, C.-Q.; Zhou, J.-Z.; Sui, Z.-Q. Protective Effects of Blueberry Anthocyanins against H2O2-Induced Oxidative Injuries in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Wang, C.; Hu, J.; Guo, X.; Zhang, D.; Wu, W.; Zhou, F.; Ji, B. Protective Effect of Quercetin and Chlorogenic Acid, Two Polyphenols Widely Present in Edible Plant Varieties, on Visible Light-Induced Retinal Degeneration In Vivo. J. Funct. Foods 2017, 33, 103–111. [Google Scholar] [CrossRef]
- Lee, H.S.; Jun, J.-H.; Jung, E.-H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells Via Suppression of MMP-9 and VEGF Activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hytti, M.; Szabó, D.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. Two Dietary Polyphenols, Fisetin and Luteolin, Reduce Inflammation but Augment DNA Damage-Induced Toxicity in Human RPE Cells. J. Nutr. Biochem. 2017, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Schwikkard, S.; Whitmore, H.; Sishtla, K.; Sulaiman, R.S.; Shetty, T.; Basavarajappa, H.D.; Waller, C.; Alqahtani, A.; Frankemoelle, L.; Chapman, A.; et al. The Antiangiogenic Activity of Naturally Occurring and Synthetic Homoisoflavonoids from the Hyacinthaceae ( sensu APGII). J. Nat. Prod. 2019, 82, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Ishihara, T.; Kaidzu, S.; Kimura, H.; Koyama, Y.; Matsuoka, Y.; Ohira, A. Protective Effect of Highly Polymeric A-Type Proanthocyanidins from Seed Shells of Japanese Horse Chestnut (Aesculus turbinata BLUME) against Light-Induced Oxidative Damage in Rat Retina. Nutrients 2018, 10, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saviranta, N.M.; Veeroos, L.; Granlund, L.J.; Hassinen, V.H.; Kaarniranta, K.; Karjalainen, R.O. Plant Flavonol Quercetin and Isoflavone Biochanin A Differentially Induce Protection Against Oxidative Stress and Inflammation in ARPE-19 Cells. Food Res. Int. 2011, 44, 109–113. [Google Scholar] [CrossRef]
- Hytti, M.; Piippo, N.; Salminen, A.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Quercetin Alleviates 4-Hydroxynonenal-Induced Cytotoxicity and Inflammation in ARPE-19 Cells. Exp. Eye Res. 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Koyama, Y.; Kaidzu, S.; Kim, Y.-C.; Matsuoka, Y.; Ishihara, T.; Ohira, A.; Tanito, M. Suppression of Light-Induced Retinal Degeneration by Quercetin via the AP-1 Pathway in Rats. Antioxidants 2019, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol Reverses the Adverse Effects of Bevacizumab on Cultured ARPE-19 Cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef] [Green Version]
- Kanavi, M.R.; Darjatmoko, S.; Wang, S.; Azari, A.A.; Farnoodian, M.; Kenealey, J.D.; Van Ginkel, P.R.; Albert, D.M.; Sheibani, N.; Polans, A.S. The Sustained Delivery of Resveratrol or A Defined Grape Powder Inhibits New Blood Vessel Formation in A Mouse Model of Choroidal Neovascularization. Molecules 2014, 19, 17578–17603. [Google Scholar] [CrossRef] [Green Version]
- Neal, S.; Buehne, K.; Besley, N.A.; Yang, P.; Silinski, P.; Hong, J.; Ryde, I.T.; Meyer, J.; Jaffe, G.J. Resveratrol Protects Against Hydroquinone-Induced Oxidative Threat in Retinal Pigment Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef] [Green Version]
- King, R.E.; Kent, K.D.; Bomser, J.A. Resveratrol Reduces Oxidation and Proliferation of Human Retinal Pigment Epithelial Cells Via Extracellular Signal-Regulated Kinase Inhibition. Chem. Interact. 2005, 151, 143–149. [Google Scholar] [CrossRef]
- Gross, S.J.; Webb, A.M.; Peterlin, A.D.; Durrant, J.R.; Judson, R.J.; Raza, Q.; Kitajewski, J.K.; Kushner, E.J. Notch Regulates Vascular Collagen IV Basement Membrane Through Modulation of Lysyl Hydroxylase 3 Trafficking. Angiogenesis 2021, 24, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Pocrnich, C.E.; Liu, H.; Feng, M.; Peng, T.; Feng, Q.; Hutnik, C.M. p38 Mitogen-Activated Protein Kinase Protects Human Retinal Pigment Epithelial Cells Exposed to Oxidative Stress. Can. J. Ophthalmol. 2009, 44, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyosseva, S.V. Targeting MAPK Signaling in Age-Related Macular Degeneration. Ophthalmol. Eye Dis. 2016, 8, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.; Markey, M.; Rapp, C.M.; Darrow, R.M.; Ziesel, A.; Organisciak, D. Enhancing the Efficacy of AREDS Antioxidants in Light-Induced Retinal Degeneration. Mol. Vis. 2017, 23, 718–739. [Google Scholar]
- Du, M.; Shen, S.; Liang, L.; Xu, K.; He, A.; Yao, Y.; Liu, S. Evaluations of the Chuanqi Ophthalmic Microemulsion In Situ Gel on Dry Age-Related Macular Degeneration Treatment. Evidence Based Complement. Altern. Med. 2020, 2020, 3805967. [Google Scholar] [CrossRef]
- Pan, H.-T.; Wang, J.-J.; Huang, J.-L.; Shuai, Y.-L.; Li, J.; Hu, Z.-Z.; Ding, Y.-Z.; Liu, Q.-H. Ranibizumab Plus Fufang Xueshuantong Capsule Versus Ranibizumab Alone for Exudative Age-Related Macular Degeneration. J. Int. Med. Res. 2020, 48, 154. [Google Scholar] [CrossRef]
- Georgiou, T.; Neokleous, A.; Nicolaou, D.; Sears, B. Pilot Study for Treating Dry Age-Related Macular Degeneration (AMD) with High-Dose Omega-3 Fatty Acids. PharmaNutrition 2014, 2, 8–11. [Google Scholar] [CrossRef]
- Cheng, Y.-P.; Ke, C.-Y.; Kuo, C.-C.; Lee, Y.-J. Effect of A Complex Lutein Formula in An Animal Model for Light-Induced Retinal Degeneration. Chin. J. Physiol. 2016, 59, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.; Wang, J. Goji Berry Effects on Macular Characteristics and Plasma Antioxidant Levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liang, L.; Snellingen, T.; Xu, K.; Gao, Y.; Zhang, F.; Guo, C.; Zuo, T.; Liang, F.; Yao, X.; et al. Mingjing Granule, A Traditional Chinese Medicine in The Treatment of Neovascular Age-Related Macular Degeneration: Study Protocol for A Randomized Controlled Trial. Trials 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues. AAPS PharmSciTech 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Zhang, M.; Zhong, Z.; Tang, L.; Feng, Y.; Wei, Z.; Li, S.; Li, Y.; Zhang, J.; Zhang, B.; et al. Ophthalmic Drops with Nanoparticles Derived from a Natural Product for Treating Age-Related Macular Degeneration. ACS Appl. Mater. Interfaces 2020, 12, 57710–57720. [Google Scholar] [CrossRef]
- Wang, S.; Cunnusamy, K. Pharmaceutical Composition for Treating Macular Degeneration (WO2012079419). Expert Opin. Ther. Patents 2013, 23, 269–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtaut, F.; Scagliarini, A.; Aires, V.; Cornebise, C.; de Barros, J.-P.P.; Olmiere, C.; Delmas, D. VEGF-R2/Caveolin-1 Pathway of Undifferentiated ARPE-19 Retina Cells: A Potential Target as Anti-VEGF-A Therapy in Wet AMD by Resvega, an Omega-3/Polyphenol Combination. Int. J. Mol. Sci. 2021, 22, 6590. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Role of Resveratrol in Transmitochondrial AMD RPE Cells. Nutrients 2020, 12, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Xu, J.; Du, X.; Cui, J.; Zhang, T.; Chen, Y. Shihu Yeguang Pill Protects Against Bright Light-Induced Photoreceptor Degeneration in Part Through Suppressing Photoreceptor Apoptosis. Biomed. Pharmacother. 2020, 126, 110050. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, S.; Angayarkanni, N. Chebulagic Acid Chebulinic Acid and Gallic Acid, The Active Principles of Triphala, Inhibit TNFα Induced Pro-Angiogenic and Pro-Inflammatory Activities in Retinal Capillary Endothelial Cells by Inhibiting p38, ERK and NFkB Phosphorylation. Vasc. Pharmacol. 2018, 108, 23–35. [Google Scholar] [CrossRef]
- Jin, M.; Dai, H.; Zhang, X.; Wang, Y.; Han, M.; Zhang, H.; Wang, Z.; Gao, X.; Li, L.; Wen, X.; et al. A Traditional Chinese Patent Medicine ZQMT for Neovascular Age- Related Macular Degeneration: A Multicenter Randomized Clinical Trial. Curr. Mol. Med. 2019, 18, 622–629. [Google Scholar] [CrossRef]
- Yang, L.; Meng, H.; Luo, D.; Deng, T.; Miao, L.; Zou, B.; Ge, X.; Hu, X.; Liu, Y.; Li, X.; et al. Inhibition of Experimental Age-Related Macular Degeneration by ZQMT in Mice. Curr. Mol. Med. 2019, 19, 434–442. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): Design Implications AREDS Report No. 1. Control. Clin. Trials 1999, 20, 573–600. [Google Scholar] [CrossRef]
- Loughman, J.; Davison, P.A.; Nolan, J.M.; Akkali, M.C.; Beatty, S. Macular Pigment and Its Contribution to Visual Performance and Experience [El pigmento macular Y Su contribución Al rendimiento Y experiencia visuales]. J. Optom. 2010, 3, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, N.; Lin, L.; Sun, E.-D.; Li, J.-D.; Li, P.-K. Macular Pigment and Serum Zeaxanthin Levels with Goji Berry Supplement in Early Age-Related Macular Degeneration. Int. J. Ophthalmol. 2018, 11, 970–975. [Google Scholar] [CrossRef]
- Chua, B.; Flood, V.; Rochtchina, E.; Wang, J.J.; Smith, W.; Mitchell, P. Dietary Fatty Acids and The 5-Year Incidence of Age-Related Maculopathy. Arch. Ophthalmol. 2006, 124, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Seddon, J.M.; George, S.; Rosner, B. Cigarette Smoking, Fish Consumption, Omega-3 Fatty Acid Intake, and Associations with Age-Related Macular Degeneration: The US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 2006, 124, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Delcourt, C.; Cristol, J.-P.; Tessier, F.; Léger, C.L.; Descomps, B.; Papoz, L. Age-Related Macular Degeneration and Antioxidant Status in The POLA Study. POLA Study Group. Pathologies Oculaires Liées à l’Age. Arch. Ophthalmol. 1999, 117, 1384–1390. [Google Scholar] [CrossRef] [Green Version]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, S.; Lee, S.-W.; Sin, H.-S.; Kim, S.-Y. Protective Effects of Fermented Paprika (Capsicum Annuum L.) on Sodium Iodate-Induced Retinal Damage. Nutrients 2020, 13, 25. [Google Scholar] [CrossRef]
- Di Marco, F.; Romeo, S.; Nandasena, C.; Purushothuman, S.; Adams, C.; Bisti, S.; Stone, J. The Time Course of Action of Two Neuroprotectants, Dietary Saffron and Photobiomodulation, Assessed in The Rat Retina. Am. J. Neurodegener. Dis. 2013, 2, 208–220. [Google Scholar]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A Longitudinal Follow-Up Study of Saffron Supplementation in Early Age-Related Macular Degeneration: Sustained Benefits to Central Retinal Function. Evid.-Based Complement. Altern. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef]
- Marangoni, D.; Falsini, B.; Piccardi, M.; Ambrosio, L.; Minnella, A.M.; Savastano, M.C.; Bisti, S.; Maccarone, R.; Fadda, A.; Mello, E.; et al. Functional Effect of Saffron Supplementation and Risk Genotypes in Early Age-Related Macular Degeneration: A Preliminary Report. J. Transl. Med. 2013, 11, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lashay, A.; Sadough, G.; Ashrafi, E.; Lashay, M.; Movassat, M.; Akhondzadeh, S. Short-Term Outcomes of Saffron Supple-Mentation in Patients with Age-Related Macular Degeneration: A Double-Blind, Placebo-Controlled, Randomized Trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 32–38. [Google Scholar] [PubMed]
- Riazi, A.; Panahi, Y.; Alishiri, A.A.; Hosseini, M.A.; Karimi Zarchi, A.A.; Sahebkar, A. The Impact of Saffron (Crocus sativus) Supplementation on Visual Function in Patients with Dry Age-Related Macular Degeneration. Ital. J. Med. 2016, 10, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.; Chang, A.A. Saffron Therapy for The Treatment of Mild/Moderate Age-Related Macular Degeneration: A Randomised Clinical Trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A Multitask Neuroprotective Agent for Retinal Degenerative Diseases. Antioxidants 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Ahmed, H.; Dixit, R.; Dharamveer; Saraf, S. Crocus sativus L.: A Comprehensive Review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, M.C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of Saffron Supplementation on Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron Supplement Maintains Morphology and Function After Exposure to Damaging Light in Mammalian Retina. Investig. Opthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Li, Z.; Glencer, P.; Cai, B.; Zhang, X.; Yang, J.; Li, X. Bringing The Age-Related Macular Degeneration High-Risk Allele Age-Related Maculopathy Susceptibility 2 Into Focus With Stem Cell Technology. Stem Cell Res. Ther. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Supanji, S.; Romdhoniyyah, D.F.; Sasongko, M.B.; Agni, A.N.; Wardhana, F.S.; Widayanti, T.W.; Prayogo, M.E.; Perdamaian, A.B.I.; Dianratri, A.; Kawaichi, M.; et al. Associations of ARMS2 and CFH Gene Polymorphisms with Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 1101–1108. [Google Scholar] [CrossRef]
- Martínez-Solís, I.; Acero, N.; Bosch-Morell, F.; Castillo, E.; González-Rosende, M.E.; Muñoz-Mingarro, D.; Ortega, T.; Sanahuja, M.A.; Villagrasa, V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Medica 2019, 85, 1292–1303. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Solís, I.; Bosch-Morell, F.; Villagrasa, V.; Ortega, T.; Acero, N.; Muñoz-Mingarro, D.; González-Rosende, M.E.; Castillo, E.; Sanahuja, M.A.; Soriano, P. Medicinal Plants and Natural Products as Neuroprotective Agents in Age-Related Macular Degeneration. Neural Regen. Res. 2020, 15, 2207–2216. [Google Scholar] [CrossRef]
- Phang, M.W.L.; Lew, S.Y.; Chung, I.; Lim, W.K.-S.; Lim, L.W.; Wong, K.H. Therapeutic Roles of Natural Remedies in Combating Hereditary Ataxia: A Systematic Review. Chin. Med. 2021, 16, 15. [Google Scholar] [CrossRef]
- John, P.A.; Wong, K.-H.; Naidu, M.; Sabaratnam, V.; David, P. Combination Effects of Curcumin and Aqueous Extract of Lignosus rhinocerotis Mycelium on Neurite Outgrowth Stimulation Activity in PC-12 Cells. Nat. Prod. Commun. 2013, 8, 711–714. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.T.; Fraunfelder, F.W. Use of Herbal Medicines and Nutritional Supplements in Ocular Disorders: An Evidence-Based Review. Drugs 2011, 71, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A. Natural Products Chemistry: The Emerging Trends and Prospective Goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Fung, F.Y.; Linn, Y.C. Steroids in Traditional Chinese Medicine: What is The Evidence? Singap. Med. J. 2017, 58, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Namdari, M.; Eatemadi, A.; Soleimaninejad, M.; Hammed, A.T. A Brief Review on The Application of Nanoparticle Enclosed Herbal Medicine for The Treatment of Infective Endocarditis. Biomed. Pharmacother. 2017, 87, 321–331. [Google Scholar] [CrossRef]
- Dubey, S.K.; Pradhan, R.; Hejmady, S.; Singhvi, G.; Choudhury, H.; Gorain, B.; Kesharwani, P. Emerging Innovations in Nano-Enabled Therapy Against Age-Related Macular Degeneration: A Paradigm Shift. Int. J. Pharm. 2021, 600, 120499. [Google Scholar] [CrossRef]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- Adijanto, J.; Philp, N.J. Cultured Primary Human Fetal Retinal Pigment Epithelium (hfRPE) as A Model for Evaluating RPE Metabolism. Exp. Eye Res. 2014, 126, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Pelkonen, L.; Sato, K.; Reinisalo, M.; Kidron, H.; Tachikawa, M.; Watanabe, M.; Uchida, Y.; Urtti, A.; Terasaki, T. LC-MS/MS Based Quantitation of ABC and SLC Transporter Proteins in Plasma Membranes of Cultured Primary Human Retinal Pigment Epithelium Cells and Immortalized ARPE19 Cell Line. Mol. Pharm. 2017, 14, 605–613. [Google Scholar] [CrossRef]
- Rimpelä, A.-K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of Melanin Binding in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Hellinen, L.; Hagström, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of Artificially Re-Pigmented ARPE-19 Retinal Pigment Epithelial Cell Model. Sci. Rep. 2019, 9, 13761. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately Differentiated ARPE-19 Cells Regain Phenotype and Gene Expression Profiles Similar to Those of Native RPE cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- Wang, H.; Hartnett, M.E. Regulation of Signaling Events Involved in The Pathophysiology of Neovascular AMD. Mol. Vis. 2016, 22, 189–202. [Google Scholar] [PubMed]
- Muraoka, Y.; Ikeda, H.O.; Nakano, N.; Hangai, M.; Toda, Y.; Okamoto-Furuta, K.; Kohda, H.; Kondo, M.; Terasaki, H.; Kakizuka, A.; et al. Real-Time Imaging of Rabbit Retina with Retinal Degeneration by Using Spectral-Domain Optical Coherence Tomography. PLoS ONE 2012, 7, e36135. [Google Scholar] [CrossRef]
- Qiu, G.; Stewart, J.M.; Sadda, S.; Freda, R.; Lee, S.; Guven, D.; de Juan, E.; Varner, S.E. A New Model of Experimental Subretinal Neovascularization in The Rabbit. Exp. Eye Res. 2006, 83, 141–152. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal Models of Age Related Macular Degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiu, G.; Tieu, E.; Munevar, C.; Wong, B.; Cunefare, D.; Farsiu, S.; Garzel, L.; Roberts, J.; Thomasy, S.M. In Vivo Multimodal Imaging of Drusenoid Lesions in Rhesus Macaques. Sci. Rep. 2017, 7, 15013. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Goody, R.; Hu, W.; Kurian, A.; James, D.; Torres, R.; Christie, L.-A.; Hohman, T.; Lawrence, M. Primate Model of Chronic Retinal Neovascularization and Vascular Leakage. Exp. Eye Res. 2020, 195, 108031. [Google Scholar] [CrossRef]
- Rho, J.; Percelay, P.; Pilkinton, S.; Hollingsworth, T.; Kornblau, I.; Jablonski, M. An Overview of Age-Related Macular Degeneration: Clinical, Pre-Clinical Animal Models and Bidirectional Translation. Anim. Models Med. Biol. 2021. [Google Scholar] [CrossRef]
- Nakano-Okuno, M.; Borah, B.R.; Nakano, I. Ethics of iPSC-Based Clinical Research for Age-Related Macular Degeneration: Patient-Centered Risk-Benefit Analysis. Stem Cell Rev. Rep. 2014, 10, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Zou, M.; Chen, A.; Zhang, Y.; Young, C.; Wang, S.; Zheng, D. Prevalence of Age-Related Macular Degeneration in Chinese Populations Worldwide: A Systematic Review and Meta-Analysis. Clin. Exp. Ophthalmol. 2019, 47, 1019–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Ayton, L.N.; Luu, C.D.; Guymer, R.H. Longitudinal Changes in Microperimetry and Low Luminance Visual Acuity in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015, 133, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Csaky, K.; Ferris, F.; Chew, E.Y.; Nair, P.; Cheetham, J.K.; Duncan, J.L. Report from The NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases. Investig. Opthalmol. Vis. Sci. 2017, 58, 3456–3463. [Google Scholar] [CrossRef]
- Terheyden, J.H.; Holz, F.G.; Schmitz-Valckenberg, S.; Lüning, A.; Schmid, M.; Rubin, G.S.; Dunbar, H.; Tufail, A.; Crabb, D.P.; Binns, A.; et al. Clinical Study Protocol for A Low-Interventional Study in Intermediate Age-Related Macular Degeneration Developing Novel Clinical Endpoints for Interventional Clinical Trials with A Regulatory and Patient Access Intention—MACUSTAR. Trials 2020, 21, 659. [Google Scholar] [CrossRef]
- Emoto, Y.; Yoshizawa, K.; Uehara, N.; Kinoshita, Y.; Yuri, T.; Shikata, N.; Tsubura, A. Curcumin Suppresses N-Methyl-N-Nitrosourea-Induced Photoreceptor Apoptosis in Sprague-Dawley Rats. In Vivo 2013, 27, 583–590. [Google Scholar]
- Chong, V. Ranibizumab for The Treatment of Wet AMD: A Summary of Real-World Studies. Eye 2016, 30, 270–286. [Google Scholar] [CrossRef]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-World Outcomes in Patients with Neovascular Age-Related Macular Degeneration Treated with Intravitreal Vascular Endothelial Growth Factor Inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef]
- Chhablani, J.K.; Sudhalkar, A.; Sethi, V.; Gogte, P.; Bondalapati, S.; Khodani, M. Retrospective Hospital-Based Analysis of Age-Related Macular Degeneration Patterns in India: 5-Year Follow-Up. Indian J. Ophthalmol. 2015, 63, 899–904. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised Controlled Trials-The Gold Standard for Effectiveness Research: Study Design: Randomised Controlled Trials. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.-A.; Held, K.S.; Viswanath, V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeau, J.H.; Auwerx, J. The Virtuous Cycle of Human Genetics and Mouse Models in Drug Discovery. Nat. Rev. Drug Discov. 2019, 18, 255–272. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Malek, G. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front. Cell Dev. Biol. 2021, 8, 24. [Google Scholar] [CrossRef] [PubMed]




| Active Compound | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
Allicin | H2O2-induced oxidative damage in human ARPE-19 cell line | 10–40 µg/mL | Protection against oxidative damage | ↑ mRNA expression and protein level of Nrf2 ↑ SOD and NQO1 (antioxidant enzyme) ↓ mRNA expression and protein level of NOX4 | [63] |
Artemisinin  | H2O2-induced oxidative damage in human D407 cell line and primary RPE cells | Various concentrations | Protection against oxidative damage and apoptosis | ↑ pAMPKα | [64] |
Astragaloside  | Isoflurane-induced apoptosis in primary RPE cells | 50 µg/mL | Protection against apoptosis | ↓ mRNA expression and protein level of CDC42, POLD1 and CCNA2 (cell cycle regulator), APH1B, APPBP2, NCSTN and APH1A (formation of β-amyloid), TRAF5 and NF-κB ↓ caspase-3/7 | [65] |
Berberine  | H2O2-induced oxidative damage in human D407 cell line and primary human RPE cells | 1 and 3 µM | Protection against oxidative damage and apoptosis | ↓ caspase-3/7 activation ↑ AMPK and total AMPK phosphorylation | [66] |
| H2O2-induced oxidative damage in human D407 cell line and primary human RPE cells | Various concentrations | Protection against oxidative damage and apoptosis | ↑ protein level of LC3B (autophagy marker) ↓ protein level of P62 (autophagy marker) ↑ AMPK and ULK1 phosphorylation ↓ mTOR phosphorylation | [67] | |
| LED light-induced retinal degeneration in BALB/c mice | 200 mg/kg, PO | Protection against retinal degeneration | ↑ mRNA expression of Rho, RPE65 and MCT3 ↓ mRNA expression of HMOX1, CP, CAT, GPx-1, SOD2 and AIF1 (oxidative damage and inflammatory marker) | [68] | |
Carnosic acid  | H2O2-induced oxidative damage in human ARPE-19 cell line and mouse photoreceptor-derived 661W cells | 10 µM | Protection against oxidative damage | ↑ mRNA expression and protein level of HO-1, NQO1, GCLM, xCT, NRF2 and SRXN1 (antioxidant enzyme) ↑ ARE activation and nuclear translocation of Nrf2 ↓ Prx2 hyperoxidation | [69] |
| Light-induced retinal degeneration in Sprague-Dawley rats | 25 mg/kg, IP | Protection against retinal degeneration | NE | ||
Celastrol  | LPS-induced inflammation in human ARPE-19 cell line | 0.05–1.5 µM | Protection against inflammation | ↑ Hsp70 ↓ IL-6 and phosphorylated NF-κB p65 (pro-inflammatory cytokine) | [70] |
Curcumin  | H2O2-induced-aging model in human ARPE-19 cell line | 10–100 µM | Protection against oxidative damage and apoptosis | ↑ Bcl-2 (anti-apoptotic protein) ↓ Bax and caspase-3 (pro-apoptotic protein) | [71] |
| H2O2-induced oxidative damage in RPE cells derived from induced pluripotent stem cells (iPSCs) obtained from patients with dry AMD | 10 μM | Protection against oxidative damage and apoptosis | ↑ mRNA expression of HO-1, SOD2, and GPx1 (antioxidant enzyme) ↓ mRNA expression of PDGF, VEGF and IGFBP-2 (oxidative stress marker) | [72] | |
| Curcuminoid Demethoxycurcumin  Bisdemethoxycurcumin Bisdemethoxycurcumin | Blue light-induced cytotoxicity in human ARPE-19 cell line | 15 μM | Protection against oxidative damage and apoptosis | ↓ mRNA expression of c-Abl and p53 (pro-apoptotic factor) | [73] |
Curcumin prodrug: Curcumin diethyl disuccinate  | H2O2-induced oxidative damage in human ARPE-19 cell line | 10 µM | Protection against oxidative damage | ↑ mRNA expression and protein level of Bcl-2, and HO-1 and NQO1 (antioxidant enzyme) ↓ mRNA expression and protein level of phosphorylated p44/42 MAPK and Bax | [74] |
Diarylheptanoid 7-(3,4 dihydroxyphenyl)-5-hydroxy-1-phenyl-(1E)-1-heptene | H2O2-induced oxidative damage in human ARPE-19 cell line | 20 µM | Protection against oxidative damage and apoptosis | NE | [75] |
Diphlorethohydroxycarmalol  | H2O2-induced oxidative damage in human ARPE-19 cell line | 25 and 50 µM | Protection against oxidative damage and apoptosis | Modulation of γH2AX and 8-OHdG (DNA damage marker) ↑ pro-caspase-9 and pro-caspase-3 (anti-apoptotic protein ↓ cytochrome c, Bax and cleaved poly (ADP-ribose) polymerase (PARP) (pro-apoptotic protein) | [76] |
FLZ  | H2O2-induced oxidative damage in human ARPE-19 cell line and primary mouse RPE cells | 1–25 µM | Protection against oxidative damage and apoptosis | ↑ Akt activation | [77] |
| TNF-α-induced inflammation in human ARPE-19 cell line | 10–50 µg/mL | Protection against inflammation | ↓ mRNA expression of ICAM-1 ↓ NF-κB p65 and phosphorylated IκBα | [78] | |
Ginsenoside | Human ARPE-19 cell line | 250 nM | Combination of ginsenoside-Rb1 and VEGF reduced the secretion of VEGF | NE | [79] |
| Human donor eyes | Various concentrations | Improvement of hydraulic and diffusional transport across Bruch’s membrane | NE | [80] | |
Glycyrrhizin  | Sodium iodate-induced oxidative damage in human ARPE-19 cell line | 20–200 µmol | Protection against oxidative damage and apoptosis | ↑ p-Akt, Nrf2 and HO-1 ↓ cleaved caspase-3 (pro-apoptotic protein) | [81] |
| Sodium iodate-induced retinal degeneration in C75BL/6 mice | 50 mg/kg, IP | Protection against retinal apoptosis | NE | ||
GPETAFLR  | H2O2-induced oxidative damage in human ARPE-19 cell line | 50 and 100 µg/mL | Protection against oxidative damage and inflammation | ↓ mRNA expression and protein level of IL-1β, IL-6, TNF-α, IFNγ and VEGF (pro-inflammatory cytokine) | [82] |
Gypenoside  | Oxidized low-density lipoprotein-induced oxidative damage in human ARPE-19 cell line | 5 µg/mL | Protection against oxidative damage and inflammation | ↑ mRNA expression and protein level of LXRα, TSPO, ABCA1, ABCG1, CYP27A1 and CYP46A1 (cholesterol metabolism and trafficking) ↓ NF-κB p65, IL-1β, IL-6, IL-8 and TNFα (inflammatory cytokine), and LDLR | [83] |
Kinsenoside | H2O2-induced oxidative damage in human ARPE-19 cell line | Various concentrations | Protection against oxidative damage and apoptosis Reduced VEGF secretion | ↓ ERK and p38 phosphorylation, VEGF and NF-κB | [84] |
Phillyrin  | H2O2-induced oxidative damage in human ARPE-19 cell line | 5–20 µM | Protection against oxidative damage and apoptosis | ↑ Bcl-2, pro-caspase-8, pro-caspase-9 and pro-caspase-3 (anti-apoptotic protein), cyclin E, CDK2, cyclin A, total Nrf2 and nuclear Nrf2 ↓ Bax, cytochrome c and Fas (pro-apoptotic protein), p53, p-p53, p21 and Keap1 | [85] |
Rosmarinic acid  | New Zealand white rabbits | 400 µg, IV implant | Protection against retinal degeneration | NE | [86] |
| Total saponins Polyphyllin I  Polyphyllin II  Polyphyllin VII  Polyphyllin H  | H2O2-induced oxidative damage in human ARPE-19 cell line | 10–40 µg/mL | Protection against oxidative damage and apoptosis | ↑ Bcl-2 (anti-apoptotic protein), Nrf2, HO-1, γ-GCS and NQO1 ↓ mRNA expression and protein level of Fas, FasL, Bax and caspase-3 (pro-apoptotic factor) | [87] |
| Carotenoid | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
β-cryptoxanthin | LED light-induced retinal degeneration in Wistar Albino rats | 2 and 4 mg/kg, PO | Protection against oxidative damage | Modulation of ATF4, ATF6, Grp78, Grp94 (mitochondrial stress marker) ↑ Bax and caspase-3 (pro-apoptotic protein), HO-1 (antioxidant enzyme), NCAM and GAP-43 ↓ IL-1β, IL-6 and NF-KB (inflammatory cytokine), Bcl-2 (anti-apoptotic protein), GFAP and VEGF | [93] |
Crocetin | TBHP-induced oxidative damage in human ARPE-19 cell line | 1–200 µM | Protection against oxidative damage | Preservation of energy production pathways ↑ ERK1/2 activation | [94] |
| Lutein and zeaxanthin Lutein  Zeaxanthin  | UVB irradiation-induced oxidative damage in human ARPE-19 cell line | 5 µM | Protection against oxidative damage | ↓ p38 MAPK and JNK1/2 phosphorylation | [95] |
| Double-blind randomized controlled trial in young healthy subjects | 10 mg/day lutein and 2 mg/day zeaxanthin | Increased serum levels of lutein and zeaxanthin; and macular pigment optical density Improvement in chromatic contrast and recovery from photostress | NE | [96] | |
Meso-zeaxanthin  | Double-blind randomized controlled trial in patients with non-advanced-stage AMD | 10 mg meso-zeaxanthin in combination with co-antioxidants | Improvement in contrast sensitivity and visual function | NE | [97] |
| Undefined carotenoids | Prospective cohort study in healthy elderly subjects | Scoring of predicted plasma carotenoid | Long term reduced risk of developing advanced-stage AMD | NE | [98] |
| Extract/ Polysaccharide | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
| Arctium lappa ethanol extract | A2E-induced cytotoxicity in human ARPE-19 cell line | 5–30 µg/mL | Protection against oxidative damage and apoptosis | ↑ Bcl-2 (anti-apoptotic protein) ↓ Bax and cleaved caspase-3 (pro-apoptotic protein) | [106] |
| White light-induced retinal degeneration in BALB/c mice | 50–200 mg/kg, PO | Protection against retinal degeneration | NE | ||
| Bilberry anthocyanin-rich aqueous extract | Light-induced retinal degeneration in pigmented rabbits | 250 and 500 mg/kg, PO | Protection against photoreceptor apoptosis | ↓ Bax, Bcl-2, and caspase-3 (pro-apoptotic protein); IL-1β and VEGF (inflammatory cytokine and angiogenic marker) | [107] |
| Bilberry ethanol extract | Blue light-emitting diode light-induced photoreceptor degeneration in murine photoreceptor (661 W) cells | 10 µg/mL | Protection against oxidative damage | ↓ LC3 autophagy marker), caspase-3/7 (pro-apoptotic protein), p38 MAPK and NF-KB activation | [108] |
| Bucida buceras ethanol extract | H2O2-induced oxidative damage in human ARPE-19 cell line | Various concentrations | Protection against oxidative damage and apoptosis | ↓ caspase-3 (pro-apoptotic protein) | [109] |
| Centella asiatica ethanol extract | MNU-induced apoptosis in human RPE-19 cell line | Various concentrations | Protection against oxidative damage and apoptosis | ↓ caspase-8, pro-caspase-9, pro-caspase-3 and pro-PARP (pro-apoptotic protein), p21 and CDK2 | [110] |
| Blue light-induced oxidative damage in human RPE cell line | Various concentrations | Protection against oxidative damage | NE | ||
| MNU-induced retinal degeneration in C57BL/6 mice | 50–100 mg/kg, PO | Protection against retinal degeneration and apoptosis | ↑ Nrf2 and HO-1 (antioxidant enzyme) ↓ caspase-3 and pro-caspase-3 (pro-apoptotic protein) | ||
| Cranberry ethyl acetate extract | Blue light-induced oxidative damage in human ARPE-19 cell line | 5–50 µg/mL | Protection against oxidative damage | NE | [72] |
| Crude fucoidan | TBHP-induced oxidative damage in human ARPE-19 cell line and primary RPE cells | 1–250 µg/mL | Reduced VEGF secretion | NE | [111] |
| Curcuma longa ethanol extract | Blue light-induced cytotoxicity in human ARPE-19 cell line | 15 μM | Protection against oxidative damage and apoptosis | ↓ mRNA expression of c-Abl and p53 (pro-apoptotic factor) | [73] |
| Diospyros kaki ethanol extract | H2O2-induced oxidative damage in immortalized rat retinal precursor cell line (R28) | Various concentrations | Protection against oxidative damage | NE | [112] |
| MNU-induced retinal degeneration in C57BL/6J mice | 10–100 mg/kg, PO | Protection against retinal degeneration | ↑ rhodopsin (retinal factor) ↓ nectin and GFAP (retinal factor), SOD1, SOD3 and GPx-1 (antioxidant enzyme) | ||
| Emblica officinalis extract | Amyloid-β-induced cellular stress in human RPE AMD transmitochondrial cybrid cells | 25 mg/mL | Protection against oxidative damage and apoptosis | ↑ mRNA expression of MT-RNR2, SOD2 and PGC-1α ↓ caspase-3/7 (pro-apoptotic protein) ↓ mRNA expression of caspase-3 (pro-apoptotic factor) and VEGF (angiogenic marker) | [113] |
Fucoidan | Human ARPE-19 cell line, primary porcine RPE cells, RPE/choroid perfusion organ culture | 100 µg/mL | Combination of fucoidan and bevacizumab reduced the secretion of VEGF and angiogenesis | ↓ VEGF165 | [114] |
| H2O2- and TBHP- induced oxidative damage in OMM-1 and human ARPE-19 cell lines, and primary porcine RPE cells | 1–100 µg/mL | Reduced VEGF secretion | NE | [115] | |
| H2O2- and TBHP- induced oxidative damage in OMM-1 and human ARPE-19 cell lines, and primary porcine RPE cells | 10 µg/mL | Reduced VEGF secretion | NE | [116] | |
| H2O2-induced oxidative damage in OMM-1 and human ARPE-19 cell lines | 1–100 µg/mL | Reduced VEGF secretion | NE | [117] | |
| Garcinia cambogia extract | CoCl2-induced HIF activation in murine retinal cone cell line (661W) and human ARPE-19 cell line | 1 mg/mL | Protection against HIF activation | ↓ mRNA expression and protein level of VEGFA, HIF-1α, BNIP3 and PDK1 (angiogenic marker and pro-apoptotic factor) | [118] |
| Laser-induced CNV in C57BL6/J mice | 0.2% extract mixed with MF diet, 30 mg/kg, IP | Protection against CNV | ↓ HIF-1α | ||
| Grape skin extract | Blue light-induced oxidative damage in human ARPE-19 cell line | 0.2–5 µg/mL | Protection against A2E oxidation, apoptosis | ↑ mRNA expression and protein level of GRP78 (ER stress and unfolded protein response marker); Bcl-2 (anti-apoptotic factor) ↓ CHOP, JNK, p-JNK, Bax, caspase-9, caspase-3, cleaved caspase-3 and cleaved caspase-9 (pro-apoptotic protein) | [119] |
Lactoferrin  | CoCl2-induced HIF activation in 661W and human ARPE-19 cell line | 1 mg/mL | Protection against HIF activation | ↓ mRNA expression of Pdk1, VEGFA and Glut1 (hypoxia response element) | [120] |
| Laser-induced CNV in C57BL6/J mice and Hif1a conditional knockout mice | 1600 mg/kg | Protection against CNV | ↓ HIF-1α | ||
| Lingonberry ethanol extract | Blue light-emitting diode light-induced photoreceptor degeneration in cultured murine photoreceptor (661 W) cells | 10 µg/mL | Protection against oxidative damage | ↓LC3 (autophagy marker), caspase-3/7 (pro-apoptotic protein), p38 MAPK and NF-KB activation | [108] |
| Lycium barbarum aqueous and ethanol extracts | UVB irradiation-induced growth arrest in human ARPE-19 cell line | 25–50 μg/mL | Protection against DNA damage and apoptosis | ↑ toll-like receptor (TLR), peroxisome proliferator-activated receptor (PPAR) and integrin activation | [121] |
| Lycium barbarum polysaccharides | H2O2-induced oxidative damage in human ARPE-19 cell line | 10–5000 µg/mL | Protection against oxidative damage and apoptosis | ↑ Bcl-2 ↓ Bax | [122] |
| Aβ1–40 oligomers-induced retinal degeneration in human ARPE-19 cell line | 3 and 14 mg/L | Protection against pyroptosis | ↓ IL-1β, IL-18, NLRP3, caspase-1 and membrane GSDMD-N (pyroptosis-related proteins) | [123] | |
| Light-induced retinal degeneration in BALB/cJ mice | 150 and 300 mg/kg, PO | Protection against photoreceptor degeneration | ↑ mRNA expression of Nrf2 and TrxR1 ↓ mRNA expression of PARP14 | [124] | |
| Melissa officinalis ethanol extract | H2O2-induced oxidative damage in human ARPE-19 cell line | 100 µg/mL | Protection against oxidative damage and apoptosis | ↑ Akt phosphorylation ↓ caspase-3/7 and PARP cleavage (pro-apoptotic protein) | [125] |
| Pueraria lobata ethanol extract | H2O2-induced oxidative damage in human ARPE-19 cell line | Various concentrations | Protection against oxidative damage | ↑ ZO-1 ↓ p38 MAPK and JNK phosphorylation | [126] |
| Red wine extract | Human ARPE-19 cell line | 30–100 µg/mL | Inhibition of VEGF-A secretion | ↓ VEGF, VEGF-A, VEGF-R2 and phosphorylated VEGF-R2 (angiogenic marker); MEK and ERK ½ phosphorylation | [127] |
| Rosemary extract | White light-induced retinal degeneration in Sprague-Dawley rats | Various concentrations, IP | Protection against retinal degeneration | ↑ HO-1 (antioxidant enzyme), rhodopsin, cone opsin, cone arrestin, retinal DNA and GFAP ↓ CEP (AMD biomarker) | [128] |
| Saudi Origanum vulgare extract-mediated gold nanoparticles | H2O2-induced oxidative damage in human RPE-19 cell line and human umbilical vein endothelial cells (HUVEC) and human RPE cells | 0.1–1 mg/mL | Protection against oxidative damage and apoptosis | ↓ mRNA expression of IL-6, TNF-α, caspase-3 and NLRP-3 (inflammatory cytokine and pro-apoptotic factor) ↓ VEGF and F4/80 | [129] |
| Solanum melongena ethanol extract | Blue light-induced oxidative damage in human RPE cell line | Various concentrations | Protection against oxidative damage | ↓ nuclear p65, CXCL8, IL-1β, RELA and PARP cleavage (inflammatory cytokine and pro-apoptotic protein) and NF-κB activation ↓ mRNA expression of CXCL8, NFKBIA, IL-1β, RELA, TRIB3 and XBPIs (inflammatory cytokine and unfolded protein response marker) | [130] |
| Blue light-induced retinal degeneration in BALB/c mice | 100 and 200 mg/kg, PO | Protection against retinal degeneration | NE | ||
| Tribulus terrestris ethanol extract | H2O2-induced oxidative damage in human RPE-19 cell line | 100 and 200 µg/mL | Protection against oxidative damage and apoptosis | ↑ mRNA expression of Nrf2, CAT, SOD1, SOD2, GST-pi, HO-1, NQO1 and GCLM ↑ Bcl-2 (anti-apoptotic factor) and Nrf2 activation ↓ Bax, cleaved caspase-3 and cleaved caspase-9 (pro-apoptotic protein) | [131] |
| Vaccinium uliginosum water extract | Blue light-induced cytotoxicity in human ARPE-19 cell line | Various concentrations | Protection against oxidative damage and apoptosis | ↓ caspase-3 and Bax/Bcl-2 ratio (pro-apoptotic protein) | [132] |
| Blue light-induced cytotoxicity in human ARPE-19 cell line | Various concentrations | Protection against oxidative damage | NE | [133] | |
| Blue light-induced retinal degeneration in BALB/c mice | 25, 50 and 100 mg/kg, PO | Protection against retinal degeneration | NE |
| Flavonoid | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
| Anthocyanin Cyanidin-3-O-glucoside  Malvidin 3-glucoside  Malvidin 3-galactoside  | UVB irradiation-induced oxidative damage in human ARPE-19 cell line | 5 µM | Protection against oxidative damage | ↓ JNK1/2 and p38 MAPK phosphorylation | [95] |
| H2O2-induced oxidative damage in human ARPE-19 cell line | 5 µg/mL | Protection against oxidative damage and apoptosis | ↑ Akt phosphorylation and Bcl-2 ↓ Erk1/2 and p38 phosphorylation; caspase-3 and Bax (pro-apoptotic protein) and VEGF | [135] | |
Chlorogenic acid | Light-induced retinal degeneration in pigmented rabbits | 39.42 mg/kg, PO | Protection against retinal inflammation | ↓ NF-κB activation | [136] |
Epigallocatechin gallate | H2O2-induced oxidative damage in human ARPE-19 cell line | 1–50 µM | Protection against ocular neovascularization and vascular permeability | ↓ mRNA expression and protein level of MMP-9, VEGF, VEGF receptor-2 and TNF-α | [137] |
| VEGF-induced vascular leakage in Sprague-Dawley rats Alkali burn-induced corneal angiogenesis in BALB/c mice | 200 mg/kg, PO | Protection against ocular neovascularization and vascular permeability | ↑ MMP-9 and platelet endothelial cell adhesion molecule (PECAM/CD31) ↓ vascular leakage and permeability | ||
Fisetin | Etoposide-induced apoptosis in human ARPE cell line and primary human RPE cells | 50 µM | Protection against inflammation | ↓ IL-8 and IL-6 (inflammatory cytokine) | [138] |
Homoisoflavonoids | Human retinal microvascular endothelial cells (HRECs) | 0.01–10 nM | Protection against angiogenesis | NE | [139] |
Kaempferol | H2O2-induced oxidative damage in human ARPE-19 cell line | 20 and 50 nM | Protection against oxidative damage and apoptosis | ↑ mRNA expression and protein level of Bcl-2 (anti-apoptotic factor) ↓ mRNA expression and protein level of Bax and caspase-3 (pro-apoptotic factor) | [140] |
| Sodium iodate-induced retinal degeneration in Sprague-Dawley rats | 3%, intravitreal, IV | Protection against retinal degeneration and apoptosis | ↑ RPE65 ↓ mRNA expression and protein level of VEGF | ||
Luteolin  | Etoposide-induced apoptosis in human ARPE cell line and primary human RPE cells | 50 µM | Protection against inflammation | ↓ IL-8 and IL-6 (inflammatory cytokine) | [138] |
Proanthocyanidins | Light-induced retinal degeneration in Sprague-Dawley rats | 30–300 mg/kg, PO | Protection against oxidative damage and apoptosis | NE | [141] |
Quercetin  | H2O2-induced oxidative damage in human ARPE-19 cell line | 100 µM | Protection against oxidative damage and inflammation | ↑ mRNA expression of Nrf2 and HO-1 ↓ mRNA expression of IL-6 and IL-1β (inflammatory cytokine) | [142] |
| 4-Hydroxynonenal-induced oxidative damage in human ARPE-19 cell line | 50 µM | Protection against inflammation | ↓ mRNA expression and protein level of IL-6, IL-8 and MCP-1 (inflammatory cytokine) ↓ p38, MAPK, ERK and CREB phosphorylation | [143] | |
| Light-induced retinal degeneration in pigmented rabbits | 33.63 mg/kg, PO | Protection against oxidative damage and inflammation | ↑ HO-1 (antioxidant enzyme) ↓ MCP-1, IL-8, IL-1β, TNF-α and COX-2 (inflammatory cytokine) | [136] | |
| Light-induced retinal degeneration in Sprague-Dawley rats | 50 mg/kg, IP | Protection against photoreceptor apoptosis and retinal degeneration | ↓ AP-1-regulated c-Jun/c-Fos heterodimerization | [144] | |
Resveratrol | Human ARPE-19 cell line | 100 µM | Combination of resveratrol and bevacizumab reduced the secretion of VEGF | ↑ mRNA expression of Notch 4 ↓ MEK1/2 (Ser217/221) and 44/42 MAPK (Thr202/Tyr204) phosphorylation and vimentin | [145] |
| Immorto mice (H-2K(b)-ts-A58(+/+) derived-choroidal endothelial cells | 100 µM | Protection against CNV | ↑ p53 (pro-apoptotic protein) ↓ Akt activation | [146] | |
| Hydroquinone-induced oxidative damage in primary human RPE cells | 15 and 30 µM | Protection against oxidative damage | ↑ mRNA expression and protein level of HO-1 and GCLC (antioxidant enzyme) ↓ XBP1 | [147] |
| Formulation | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
| AREDS and rosemary/carnosic acid/ursolic acid | Light-induced retinal degeneration in Sprague-Dawley rats | 17 mg/kg, IP | Protection against retinal degeneration | ↑ mRNA expression of EGR1, GNG11, RGD1564999, SCN7A, Olr425, Vom2r65, OPRK (retinal factor) ↑ HO-1 antioxidant enzyme) rhodopsin, rod S-antigen, cone opsin and cone arrestin ↓ CEP (AMD biomarker) | [152] |
| AREDS2 | Double-blind randomized controlled trial in healthy elderly subjects | Dose-ranging, PO | No effect on reducing the risk of progression to advanced AMD | NE | [104] |
| Chuanqi microemulsion in situ gel | Sodium iodide-induced retinal degeneration in Sprague Dawley rats | 20 μL, dripping | Protection against retinal degeneration | NE | [153] |
| Curcumin supplement | Retrospective case-control study in patients with neovascular AMD | NE | Combination of curcuma and anti-VEGF reduced the frequency of injections | NE | [91] |
| Fufang Xueshuantong | Prospective randomized controlled pilot study in patients with CNV | 4500 mg/day, PO | Combination of Fufang Xueshuantong with ranibizumab reduced the CNV-PED complex thickness Improvement in BCVA | NE | [154] |
| Liquid formulation of omega-3 concentrate | Open-label pilot study in patients with dry AMD | 3.4g of eicosapentaenoic acid (EPA) and 1.6g of docosahexaenoic acid (DHA), PO | Improvement in vision | NE | [155] |
| Lutein formulation | Light-induced retinal degeneration in Sprague-Dawley rats | 104 mg/kg, PO | Protection against photoreceptor apoptosis and retinal degeneration | NE | [156] |
| Milk-based formulation of Lycium barbarum | Double-blind randomized controlled trial in healthy elderly subjects | 13.7 g, PO | Protection against macula hypopigmentation and accumulation of soft drusen | NE | [157] |
| Mingjing | Double-blind randomized controlled trial in patients with neovascular AMD | 5.95 g, PO | Combination of Mingjing and ranibizumab reduced the frequency of injections | NE | [158] |
| Nanomicellar drop | H2O2-induced oxidative damage in human D407 cell line | 10 µM | Protection against oxidative damage | ↓ VEGF | [159] |
| Ophthalmic drop formulation | Laser radiation-induced in a nonhuman primate model of AMD-rhesus monkey | 1 mg/mL, dripping | Promotion of autophagy and suppression of angiogenesis | ↑ 1,25D3-MARRS | [160] |
| Pharmaceutical composition (Patent No: WO2012079419) | Light-induced retinal CNV in Brown Norway rats | NA | Protection against CNV | NE | [161] |
| Clinical trial in patients with neovascular CNV | NA | Protection against CNV | NE | ||
| RESVEGA® | Human ARPE-19 cell line | Various concentrations | Inhibition of VEGF-A secretion | ↓ VEGF-R2/Cav-1 complex dissociation into lipid rafts, and MAPK activation | [162] |
| Resveratrol formulation | Human RPE AMD transmitochondrial cybrid cells | 1000 µM | Protection against oxidative damage | NE | [163] |
| Shihu Yeguang | Bright light-induced photoreceptor degeneration in BALB/c mice | 57 mg/20 g, PO | Protection against retinal degeneration and apoptosis | ↑ Bcl-2 (anti-apoptotic factor) ↓ mRNA expression of c-fos and c-jun (pro-apoptotic factor); TNF-α (pro-inflammatory cytokine) | [164] |
| Triphala | TNF-α-induced angiogenesis and inflammation in rhesus monkey choroidal-retinal endothelial cell line (RF/6A) | Various concentrations | Protection against inflammation, tube formation, chemotaxis and proliferation | ↑ IL-10 and IL-13 (inflammatory cytokine) ↓ MMP-9; p38, ERK and NF-κB phosphorylation ↓ mRNA expression of IL-6, IL-8, eotaxin, MCP-1, MIP-1β, RANTES, IL-5 and PDGF-BB (inflammatory cytokine) | [165] |
| ZQMT | Randomized clinical trial in patients with CNV | 15 tablets, PO | Improvement in visual acuity Combination of ZQMT and ranibizumab reduced the frequency of injections | NE | [166] |
| Laser-induced CNV in Crb1rd8 mice | 25 mg/mL, PO | Protection against AMD-related retinopathy | ↑ CCL2 and CX3CR1 (chemokine axis) activation | [167] |
| Vitamin | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
Vitamin B6 | CoCl2-induced hypoxic condition in mouse photoreceptor-derived 661W and human ARPE-19 cell lines | 1 mg/mL | Suppression of retinal neovascularization | ↓ mRNA expression of VEGF ↓ HIF | [174] |
| Light-induced retinal degeneration in C57BL/6 and BALB/c mice | 9 and 35 mg/kg, PO | Suppression of retinal neovascularization | ↓ HIF |
| Whole Food | Model | Concentration/Dose | Finding | Mode of Action | Reference |
|---|---|---|---|---|---|
| Defined grape powder | Laser-induced CNV in C57BL/6J mice | 100 mg/animal, PO | Protection against CNV | NE | [146] |
| Fermented Capsicum annuum | Sodium iodate-induced oxidative damage in human ARPE-19 cell line | 500 µg/mL | Protection against oxidative damage and apoptosis | ↓ cleaved PARP-1, caspase-8 and caspase-3 (pro-apoptotic factor); AKT, JNK and p38 phosphorylation | [175] |
| Sodium iodate-induced retinal degeneration in C57BL/6 mice | 195 mg/kg, PO | Protection against retinal degeneration | |||
| Lycium barbarum | Double-blind randomized controlled trial in patients with neovascular AMD | 25 g/day, PO | Improvement in macular pigment optical density | NE | [170] |
| Rice bran | CoCl2-induced hypoxic condition in mouse photoreceptor-derived 661W and human ARPE-19 cell lines | 1 mg/mL | Suppression of retinal neovascularization | ↓ HIF ↓ mRNA expression of VEGF | [174] |
| Light-induced retinal degeneration in C57BL/6 and BALB/c mice | 587.5 mg/kg, PO | Suppression of retinal neovascularization | ↓ HIF | ||
| Rosemary oil | White light-induced retinal degeneration in Sprague-Dawley rats | Various concentrations, IP | Protection against retinal degeneration | ↑ HO-1 (antioxidant enzyme), rhodopsin, cone opsin, cone arrestin, retinal DNA and GFAP ↓ CEP (AMD biomarker) | [128] |
| Saffron | Light-induced retinal degeneration in Sprague-Dawley rats | 1 mg/kg, PO | Protection against photoreceptor degeneration | NE | [176] |
| Open-label longitudinal study in patients with AMD | 20 mg/day, PO | Improvement in macular function in early/moderate-stage AMD | NE | [177] | |
| Open-label longitudinal study in patients with AMD | 20 mg/day, PO | Improvement in macular function | NE | [178] | |
| Double-blind randomized controlled trial in patients with AMD | 30 mg/day, PO | Improvement in retinal function in advanced-stage AMD | NE | [179] | |
| Clinical trial in patients with dry AMD | 50 mg/day, PO | Improvement in visual function Delaying the progression of dry AMD | NE | [180] | |
| Double-blind randomized controlled trial in patients with AMD | 20 mg/day, PO | Preservation of retinal function in mild/moderate-stage AMD | NE | [181] | |
| Saffron (Patent: W02015/145316) | Light-induced retinal degeneration in albino rats | 1 mg/kg | Protection against photoreceptor apoptosis and retinal degeneration | ↓MMP-3 | [182] |
| Clinical trial in patients with AMD | NE | Delaying the progression of AMD | NE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.-H.; Nam, H.-Y.; Lew, S.-Y.; Naidu, M.; David, P.; Kamalden, T.A.; Hadie, S.N.H.; Lim, L.-W. Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals 2022, 15, 101. https://doi.org/10.3390/ph15010101
Wong K-H, Nam H-Y, Lew S-Y, Naidu M, David P, Kamalden TA, Hadie SNH, Lim L-W. Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals. 2022; 15(1):101. https://doi.org/10.3390/ph15010101
Chicago/Turabian StyleWong, Kah-Hui, Hui-Yin Nam, Sze-Yuen Lew, Murali Naidu, Pamela David, Tengku Ain Kamalden, Siti Nurma Hanim Hadie, and Lee-Wei Lim. 2022. "Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review" Pharmaceuticals 15, no. 1: 101. https://doi.org/10.3390/ph15010101
APA StyleWong, K. -H., Nam, H. -Y., Lew, S. -Y., Naidu, M., David, P., Kamalden, T. A., Hadie, S. N. H., & Lim, L. -W. (2022). Discovering the Potential of Natural Antioxidants in Age-Related Macular Degeneration: A Review. Pharmaceuticals, 15(1), 101. https://doi.org/10.3390/ph15010101