A Comprehensive Study of Therapeutic Applications of Chamomile
Abstract
:1. Introduction
2. Morphology
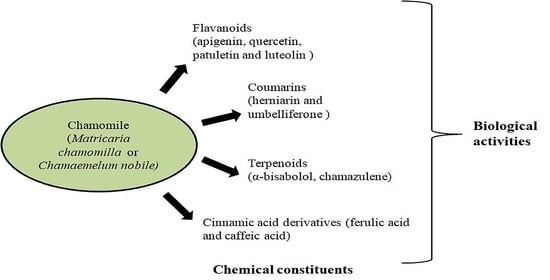
3. Chemical Constituents
4. Biological Activities of Chamomile
4.1. Anti-Inflammatory Activity
4.2. Antioxidant Activity
4.3. Anti-Allergic Activity
4.4. Anti-Microbial Activity
4.5. Analgesic Activity
4.6. Anti-Cancer Activity
4.7. Central-Nervous-System-Related Disorders
4.8. Anti-Hypertensive Activity
4.9. Hepatoprotectiveproperties
4.10. Protective Effects on Metabolic Syndrome
4.11. Other Therapeutic Applications
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bansal, P.; Gupta, V.; Mittal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of Matricaria recutita—A review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Chamomile. In Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesda, MD, USA, 2021.
- Šalamon, I. The Slovak gene pool of German chamomile (Matricaria recutita L.) and comparison in its parameters. Hortic. Sci. 2018, 31, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.; Singh, S.; Kumar, V.; Kumar, A.; Kumari, A.; Rathore, S.; Kumar, R.; Singh, S. A Comprehensive review on biology, genetic improvement, agro and process technology of German Chamomile (Matricaria chamomilla L.). Plants 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic profile by HPLC-PDA-MS of Greek chamomile populations and commercial varieties and their antioxidant activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Moghaddasi Mohammad, S. Study on Cammomile(Matricaria chamomilla L.) usage and farming. Adv. Environ. Biol. 2011, 5, 1446–1453. [Google Scholar]
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Antibacterial effect of ginger (zingiberofficinale) roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient. J. Chem. 2016, 32, 817–837. [Google Scholar] [CrossRef] [Green Version]
- Hameed, I.H.; Hussein, H.M.; Ohammed, U.; Jenan, M. Determination of bioactive chemical composition of callosobruchusmaculutus and investigation of its anti-fungal activity Toxicity. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1293–1299. [Google Scholar]
- Andreucci, A.C.; Ciccarelli, D.; Desideri, I.; Pagni, A.M. Glandular hairs and secretory ducts of Matricaria chamomilla (Asteraceae): Morphology and histochemistry. Ann. Bot. Fennici 2008, 45, 11–18. [Google Scholar] [CrossRef]
- Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2016, 8, 8–24. [Google Scholar]
- Pino, J.A.; Bayat, F.; Marbot, R.; Aguero, J. Essential oil of chamomile Chamomilla recutita (L.) Rausch. From Iran. J. Essent. Oil Res. 2002, 14, 407–408. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative analysis of phenolic composition of six commercially available chamomile (Matricaria chamomilla L.) extracts: Potential biological implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vinvieri, F.F.; Prucher, D. Characterisation of M. recutita L. flower extract by HPLCMS and HPLC-DAD analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Chauhan, E.S.; Aishwarya, J. Nutraceutical analysis of Marticariarecutita (Chamomile) dried leaves and flower powder and comparison between them. Int. J. Phytomed. 2018, 10, 111–114. [Google Scholar] [CrossRef]
- Matos, F.J.A.; Machado, M.I.L.; Alencar, J.W.; Craveiro, A.A. Constituents of brazilian chamomile oil. J. Essent. Oil Res. 2011, 5, 337–339. [Google Scholar] [CrossRef]
- Ramadan, M.; Goeters, S.; Watzer, B.; Krause, E.; Lohmann, K.; Bauer, R.; Hempel, B.; Imming, P. Chamazulene carboxylic acid and matricin: A natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J. Nat. Prod. 2006, 69, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Vienna, C.F.; Graz, R.B.; Hohenheim, R.C.; Milano, D.T.; Trieste, A.T.; Wien, K.Z. Study on the assessment of plants/herbs, plant/herb extracts and their naturally or synthetically produced components as ‘additives’ for use in animal production. EFSA Support. Publ. 2017, 4, 070828. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.; Hou, X.; Liu, C.; Sun, J.; Guo, C.; Su, L.; Jiang, W.; Ling, C.; Wang, C.; Wang, H.; et al. Phytochemical and comparative transcriptome analyses reveal different regulatory mechanisms in the terpenoid biosynthesis pathways between Matricaria recutita L. and Chamaemelumnobile L. BMC Genom. 2020, 21, 169. [Google Scholar] [CrossRef] [Green Version]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res. Pharm. Sci. 2014, 9, 31–37. [Google Scholar]
- Antonelli, A.; Fabbri, C. Study on Roman chamomile (Chamaemelumnobile L. All.) Oil. J. Essent. Oil Res. 1998, 10, 571–574. [Google Scholar] [CrossRef]
- Ling, C.; Zheng, L.; Yu, X.; Wang, H.; Wang, C.; Wu, H.; Zhang, J.; Yao, P.; Tai, Y.; Yuan, Y. Cloning and functional analysis of three aphid alarm pheromone genes from German chamomile (Matricaria chamomilla L.). Plant Sci. 2020, 294, 110463. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.; Fan, Y. Volatile terpenoids: Multiple functions, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 2010, 11, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-inflammatory and barrier-stabilising effects of Myrrh, Coffee charcoal and Chamomile flower extract in a co-culture cell model of the intestinal mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile: An anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar]
- Fan, X.; Du, K.; Li, N.; Zheng, Z.; Qin, Y.; Liu, J.; Sun, R.; Su, Y. Evaluation of anti-nociceptive and anti-inflammatory effect of Luteolin in mice. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 351–364. [Google Scholar] [CrossRef]
- Flemming, M.; Kraus, B.; Rascle, A.; Jürgenliemk, G.; Fuchs, S.; Fürst, R.; Heilmann, J. Revisited anti-inflammatory activity of matricine in vitro: Comparison with chamazulene. Fitoterapia 2015, 106, 122–128. [Google Scholar] [CrossRef]
- Maurya, A.K.; Singh, M.; Dubey, V.; Srivastava, S.; Luqman, S.; Bawankule, D.U. α-(-)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr. Pharm. Biotechnol. 2014, 15, 173–181. [Google Scholar] [CrossRef]
- Tomić, M.; Popović, V.; Petrović, S.; Stepanović-Petrović, R.; Micov, A.; Pavlović-Drobac, M.; Couladis, M. Antihyperalgesic and antiedematous activities of bisabolol-oxides-rich matricaria oil in a rat model of inflammation. Phytother. Res. 2014, 28, 759–766. [Google Scholar] [CrossRef]
- Wang, W.; Yue, R.F.; Jin, Z.; He, L.M.; Shen, R.; Du, D.; Tang, Y.Z. Efficiency comparison of apigenin-7-O-glucoside and trolox in antioxidative stress and anti-inflammatory properties. J. Pharm. Pharmacol. 2020, 72, 1645–1656. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Chandrashekhar, V.M.; Halagali, K.S.; Nidavani, R.B.; Shalavadi, M.H.; Biradar, B.S.; Biswas, D.; Muchchandi, I.S. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J. Ethnopharmacol. 2011, 137, 336–340. [Google Scholar] [CrossRef]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016, 95, 693. [Google Scholar]
- Kazemian, H.; Ghafourian, S.; Sadeghifard, N.; Houshmandfar, R.; Badakhsh, B.; Taji, A.; Shavalipour, A.; Mohebi, R.; Ebrahim-Saraie, H.S.; Houri, H.; et al. In vivo antibacterial and wound healing activities of Roman chamomile (Chamaemelumnobile). Infect. Disord. Drug Targets 2018, 18, 41–45. [Google Scholar] [CrossRef]
- Kazemian, H.; Ghafourian, S.; Heidari, H.; Amiri, P.; Yamchi, J.K.; Shavalipour, A.; Houri, H.; Maleki, A.; Sadeghifard, N. Antibacterial, anti-swarming and anti-biofilm formation activities of Chamaemelumnobile against Pseudomonas aeruginosa. Rev. Soc. Bras. Med. Trop. 2015, 48, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Gamze, G.; Betül, D.; Sinem, I.; Fatih, D. Antimicrobial and toxicity profiles evaluation of the Chamomile (Matricariarecutita L.) essential oil combination with standard antimicrobial agents. Ind. Crops Prod. 2018, 120, 279–285. [Google Scholar] [CrossRef]
- Oliveira Ribeiro, S.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and cytotoxic activities of ten commercially available essential Oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef]
- Wagih Abd ElFattah, E.; El, A. Antimicrobial activity of chamomile acetone extract against some experimentally-induced skin infections in mice. Egypt. J. Environ. Res. 2014, 2, 58–70. [Google Scholar]
- Koch, C.; Reichling, J.; Kehm, R.; Sharaf, M.M.; Zentgraf, H.; Schneele, J.; Schnitzler, P. Efficacy of anise oil, dwarf-pine oil and chamomile oil against thymidine-kinase-positive and thymidine-kinase-negative herpesviruses. J. Pharm. Pharmacol. 2008, 60, 1545–1550. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Aboonq, M.S.; El Rashedy, A.G.; Aljehani, Y.T.; Abou El-Magd, R.M.; Okashah, A.M.; El-Anzi, M.E.; Alharbi, M.B.; El-Tahlawi, R.; Nabo, M.; et al. Promising preventive and therapeutic effects of TaibUVID nutritional supplements for COVID-19 pandemic: Towards better public prophylaxis and treatment (A retrospective study). Am. J. Blood Res. 2020, 10, 266–282. [Google Scholar]
- Chaves, P.; Hocayen, P.; Dallazen, J.L.; de Paula Werner, M.F.; Iacomini, M.; Andreatini, R.; Cordeiro, L. Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects. Int. J. Biol. Macromol. 2020, 164, 1675–1682. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Gupta, S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agric. Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef]
- Viola, H.; Wasowski, C.; Levi de Stein, M.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.H.; Paladini, A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Silveira, V.; Santos Rubio, K.T.; PoletiMartucci, M.E. Anxiolytic effect of Anthemisnobilis L. (roman chamomile) and Citrus reticulata Blanco (tangerine) essential oils using the light-dark test in zebrafish (Danio rerio). J. Ethnopharmacol. 2022, 298, 115580. [Google Scholar] [CrossRef]
- Staufenbiel, S.M.; Penninx, B.W.; Spijker, A.T.; Elzinga, B.M.; van Rossum, E.F. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 2013, 38, 1220–1235. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Carvalho, J.P.; Tyrka, A.R.; Wier, L.M.; Mello, A.F.; Mello, M.F.; Anderson, G.M.; Wilkinson, C.W.; Price, L.H. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 2007, 62, 1080–1087. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamura, K.; Furukawa, K.; Konishi, Y.; Ogino, T.; Higashiura, K.; Yago, H.; Okamoto, K.; Otsuka, M. A new nonpeptide tachykinin NK1 receptor antagonist isolated from the plants of Compositae. Chem. Pharm. Bull. 2002, 50, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Miura, T.; Mimaki, Y.; Sashida, Y. Effect of inhalation of chamomile oil vapour on plasma ACTH level in ovariectomized-rat under restriction stress. Biol. Pharm. Bull. 1996, 19, 1244–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsterdam, J.D.; Li, Q.S.; Xie, S.X.; Mao, J.J. Putative antidepressant effect of chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J. Altern. Complement. Med. 2020, 26, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, P.; FahanikBabaei, J.; Vazifekhah, S.; Nikbakht, F. Evaluation of the neuroprotective, anticonvulsant, and cognition-improvement effects of apigenin in temporal lobe epilepsy: Involvement of the mitochondrial apoptotic pathway. Iran. J. Basic Med. Sci. 2019, 22, 752–758. [Google Scholar] [PubMed]
- Kim, M.; Jung, J.; Jeong, N.Y.; Chung, H.J. The natural plant flavonoid apigenin is a strong antioxidant that effectively delays peripheral neurodegenerative processes. Anat. Sci. Int. 2019, 94, 285–294. [Google Scholar] [CrossRef]
- Awaad, A.A.; El-Meligy, R.M.; Zain, G.M.; Safhi, A.A.; Al Qurain, N.A.; Almoqren, S.S.; Zain, Y.M.; Sesh Adri, V.D.; Al-Saikhan, F.I. Experimental and clinical antihypertensive activity of Matricaria chamomilla extracts and their angiotensin-converting enzyme inhibitory activity. Phytother. Res. 2018, 32, 1564–1573. [Google Scholar] [CrossRef]
- Gao, H.L.; Yu, X.J.; Hu, H.B.; Yang, Q.W.; Liu, K.L.; Chen, Y.M.; Zhang, Y.; Zhang, D.D.; Tian, H.; Zhu, G.Q.; et al. Apigenin improves hypertension and cardiac hypertrophy through modulating NADPH oxidase-dependent ROS generation and cytokines in hypothalamic paraventricular nucleus. Cardiovasc. Toxicol. 2021, 21, 721–736. [Google Scholar] [CrossRef]
- Shebbo, S.; El Joumaa, M.; Kawach, R.; Borjac, J. Hepatoprotective effect of Matricaria chamomilla aqueous extract against 1,2-Dimethylhydrazine-induced carcinogenic hepatic damage in mice. Heliyon 2020, 6, 04082. [Google Scholar] [CrossRef]
- Sebai, H.; Jabri, M.A.; Souli, A.; Hosni, K.; Rtibi, K.; Tebourbi, O.; El-Benna, J.; Sakly, M. Chemical composition, antioxidant properties and hepatoprotective effects of chamomile (Matricariarecutita L.) decoction extract against alcohol-induced oxidative stress in rat. Gen. Physiol. Biophys. 2015, 34, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Bayliak, M.M.; Dmytriv, T.R.; Melnychuk, A.V.; Strilets, N.V.; Storey, K.B.; Lushchak, V.I. Chamomile as a potential remedy for obesity and metabolic syndrome. EXCLI J. 2021, 20, 1261–1286. [Google Scholar]
- Zhao, J.; Khan, S.I.; Wang, M.; Vasquez, Y.; Yang, M.H.; Avula, B.; Wang, Y.H.; Avonto, C.; Smillie, T.J.; Khan, I.A. Octulosonic acid derivatives from Roman chamomile (Chamaemelumnobile) with activities against inflammation and metabolic disorder. J. Nat. Prod. 2014, 77, 509–515. [Google Scholar] [CrossRef]
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Valladares, B.; Pinero, J.E.; Lorenzo-Morales, J.; Akkari, H. Amoebicidal activity of α-bisabolol, the main sesquiterpene in chamomile (Matricariarecutita L.) essential oil against the trophozoite stage of Acanthamoeba castellani Neff. Acta Parasitol. 2017, 62, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Hajaji, S.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Jiménez, I.A.; Bazzocchi, I.L.; Valladares, B.; Akkari, H.; Lorenzo-Morales, J.; Piñero, J.E. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 2018, 117, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takahashi, R.; Ogino, F. Antipruritic effect of the single oral administration of German chamomile flower extract and its combined effect with antiallergic agents in ddY mice. J. Ethnopharmacol. 2005, 101, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, Z.B.; Beiranvand, S.P.; Bokaie, M. Efficacy of Chamomile in the treatment of premenstrual syndrome: A systematic review. J. Pharmacopunct. 2019, 22, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Goos, K.H.; Arnhold, J.; Nieber, K. Mechanisms on spasmolytic and anti-inflammatory effects of a herbal medicinal product consisting of myrrh, chamomile flower, and coffee charcoal. Spasmolytische und antiinflammatorischeWirkmechanismeneinespflanzlichenArzneimittelsbestehendausMyrrhe, Kamillenblüten und Kaffeekohle. Wien. Med. Wochenschr. Suppl. 2017, 167, 169–176. [Google Scholar]
- Sampaio, T.L.; Menezes, R.R.; da Costa, M.F.; Meneses, G.C.; Arrieta, M.C.; Chaves Filho, A.J.; de Morais, G.B.; Libório, A.B.; Alves, R.S.; Evangelista, J.S.; et al. Nephroprotective effects of (-)-α-bisabolol against ischemic-reperfusion acute kidney injury. Phytomedicine 2016, 23, 1843–1852. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Jalali, A.; Keshavarz, P. Effect of chamomile hydroalcoholic extract on Bleomycin-induced pulmonary fibrosis in rat. Tanaffos 2018, 17, 264–271. [Google Scholar]
- El-Salamouni, N.S.; Ali, M.M.; Abdelhady, S.A.; Kandil, L.S.; Elbatouti, G.A.; Farid, R.M. Evaluation of chamomile oil and nanoemulgels as a promising treatment option for atopic dermatitis induced in rats. Expert Opin. Drug Deliv. 2020, 17, 111–122. [Google Scholar] [CrossRef]
- Atar, Y.; Karaketir, S.; Aydogdu, I.; Sari, H.; Bircan, H.S.; Uyar, Y.; Ekincioglu, E.; Karaketir, S.G.; Atac, E.; Berkiten, G. Comparison of isotonic seawater nasal spray containing chamomile liquid extract and other isotonic seawater nasal washing solutions for allergic rhinitis. Ann. Otol. Rhinol. Laryngol. 2022, 131, 427–434. [Google Scholar] [CrossRef]
- Elhadad, M.A.; El-Negoumy, E.; Taalab, M.R.; Ibrahim, R.S.; Elsaka, R.O. The effect of topical chamomile in the prevention of chemotherapy-induced oral mucositis: A randomized clinical trial. Oral Dis. 2022, 28, 164–172. [Google Scholar] [CrossRef]
- Morales-Bozo, I.; Ortega-Pinto, A.; Rojas Alcayaga, G.; Aitken Saavedra, J.P.; Salinas Flores, O.; Lefimil Puente, C.; Lozano Moraga, C.; Manríquez Urbina, J.M.; Urzúa Orellana, B. Evaluation of the effectiveness of a chamomile (Matricaria chamomilla) and linseed (Linumusitatissimum) saliva substitute in the relief of xerostomia in elders. Gerodontology 2017, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ebada, M.E. Essential oils of green cumin and chamomile partially protect against acute acetaminophen hepatotoxicity in rats. An. Acad. Bras. Cienc. 2018, 90, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Zardosht, R.; Basiri, A.; Sahebkar, A.; Emami, S.A. Effect of chamomile oil on cesarean section pain in primiparous women: A randomized clinical trial. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; Dias, F.; Costa, G.; Campos, M. Chamomile reveals to be a potent galactogogue: The unexpected effect. J. Matern. Fetal Neonatal. Med. 2018, 31, 116–118. [Google Scholar] [CrossRef]
- Farideh, Z.Z.; Bagher, M.; Ashraf, A.; Akram, A.; Kazem, M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010, 11, 169–174. [Google Scholar]


| Chemical Component | German Chamomile (% w/w) | Roman Chamomile (% w/w) |
|---|---|---|
| Esters | 0.28 | 75 |
| Aliphatic aldehydes | 0.25 | 2 |
| Ketones | 0.5 | 3 |
| Sesquiterpenes | 35 | 3 |
| Lactones and coumarins | 9 | 2 |
| Monoterpenes | 1 | 5 |
| Alcohols | 20 | 5 |
| Apigenin and its derivatives | 0.39 | 0.12 |
| Total flavonoid content | 0.82 | 0.16 |
| Formulation | Activity | Disease | Study | Inference | Reference |
|---|---|---|---|---|---|
| Hydroalcoholic extract | Anti-inflammatory, antioxidant | Pulmonary fibrosis | Invitro | Different doses of chamomile extract were given (400, 600, 800, 1000, and 1500 mg/kg/day) to bleomycin-induced pulmonary fibrosis. 1500 mg/kg/day significantly reduced the damage to lungs. | [68] |
| Methanolic and aqueous extract | Antioxidant | Anti-helmintic | Invitro | IC50 values exhibited by methanolic and aqueous extracts against worms are 1.559 mg/mL and 2.559 mg/mL, respectively. The mortality rate of eggs shown by Albendazole standard was 91.75%, whereas, by chamomile extracts, it was 100% with a 0% recovery rate. | [63] |
| Topical nanoemulgel | Antioxidant, anti-inflammatory | Atopic dermatitis | In-vivo | The severity of lesions induced by capsaicin administration significantly reduced after the topical application of chamomile oil and nanogel. Furthermore, inflammatory markers, IL-4 and IL-22, and levels of nitric oxide also reduced considerably in the treatment group. Uponcomparison, it can be seen that nanoemulgel formulations showed better results than oil due to improved skin retention and penetration. | [69] |
| Nasal spray containing chamomile extracts | Antioxidant | Allergic rhinitis | Clinical trial | The antiallergy effects were evaluated on the basis of Sino-Nasal Outcome Test (SNOT) scores. A significant decrease in SNOT scores was observed in patients when treated with nasal sprays of isotonic seawater containing chamomile oil and steroid (mometasone furoate) compared to treatments comprising isotonic seawater with steroid, hypervolume seawater and steroid, and only steroid. Similar results were obtained for nasal mucociliary clearance time, which was significantly reduced. | [70] |
| Topical gel of chamomile alcoholic extract (3%) | Anti-inflammatory, analgesic | Chemotherapy induced oral mucositis | Clinical trial | The pain severity was assessed by numeric rating scale (NRS) every week for 21 days. Treatment with chamomile showed lower NRS scores compared to topical miconaz gel. | [71] |
| Oral mixture of linseed mucilage and chamomile flower decoction | Not known | Xerostomia | Clinical trial | The trial was conducted on 74 aged patients experiencing xerostomia. A proportion of participants (59.5%) felt the sensation of thick saliva at the end of the study period. The results were statistically significant (p < 0.05) compared to conventional saliva substitutes. | [72] |
| Oral chamomile oil and cumin oil | Antioxidant, hepatoprotective | Acetaminophen induced hepatotoxicity | In-vivo | The protective effects of cumin oil and chamomile oil were compared. Chamomile oil exhibited moderate protective effects as evident by decreased glutathione and superoxide dismutase activity in livers. | [73] |
| Chamomile extract in capsules | Anxiolytic effect | Generalized anxiety disorder (GAD) | Clinical trial | The effects were tested on 179 subjects with GAD, and Hamilton rating scalefor anxiety and depression was determined. All subjects showed antidepressant effects upon chamomile treatments, which suggests its possible role in anxiety and depression. | [53] |
| Chamomile oil drops | Analgesic | Cesarean Section Pain | Clinical trial | Pregnant women numbering 128 participated in the study. The subjects inhaled drops at 4, 8, and 12 h after surgery and the pain intensity was measured using visual analog scale. The findings indicated that chamomile oil significantly reduced the severity of pain in women compared to placebo drops. | [74] |
| Aqueous chamomile infusion | Estrogenic activity | Galactogogue | Case study | A 29-year-old woman started consuming chamomile infusion after the birth of a baby. After 3 months, milk production enhanced from 60 mL to 90 mL. | [75] |
| Alcoholic chamomile extract | Polycystic ovary syndrome | In-vitro | The production of cysts in the ovaries was stimulated by giving estradiol valerate injections. Subsequently, rats were treated with alcoholic chamomile extract (50 mg/kg) or corn oil only (control). It was seen that the cysts were reduced drastically after chamomile treatment. The levels of luteinizing hormone and follicle stimulating hormone also decreased considerably. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. https://doi.org/10.3390/ph15101284
Sah A, Naseef PP, Kuruniyan MS, Jain GK, Zakir F, Aggarwal G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals. 2022; 15(10):1284. https://doi.org/10.3390/ph15101284
Chicago/Turabian StyleSah, Amit, Punnoth Poonkuzhi Naseef, Mohammed S. Kuruniyan, Gaurav K. Jain, Foziyah Zakir, and Geeta Aggarwal. 2022. "A Comprehensive Study of Therapeutic Applications of Chamomile" Pharmaceuticals 15, no. 10: 1284. https://doi.org/10.3390/ph15101284
APA StyleSah, A., Naseef, P. P., Kuruniyan, M. S., Jain, G. K., Zakir, F., & Aggarwal, G. (2022). A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals, 15(10), 1284. https://doi.org/10.3390/ph15101284