Optimized Therapeutic 177Lu-Labeled PSMA-Targeted Ligands with Improved Pharmacokinetic Characteristics for Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Radiochemical Synthesis and Quality Control
2.2. Partition Coefficient and Stability
2.3. In Vitro Cellular Studies
2.4. Pharmacokinetics and Biodistribution Studies
2.5. Small-Animal SPECT
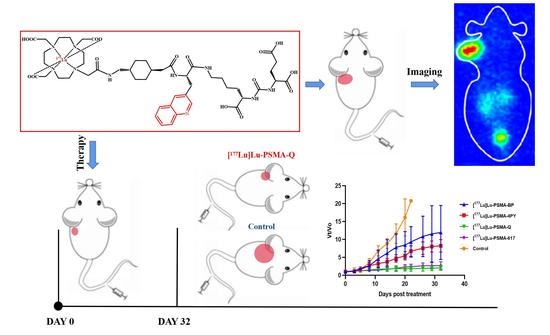
2.6. Therapy Study
2.7. Radiotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis, Radiolabeling and Quality Control
4.2. Partition Coefficient
4.3. In Vitro Stability
4.4. Cell Lines and Culture Condition
4.5. Cell Binding and Internalization Studies
4.6. Pharmacokinetics
4.7. Tumor Models
4.8. Biodistribution
4.9. Small-Animal SPECT
4.10. Therapy Study
4.11. Radiotoxicity
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miller, D.C.; Hafez, K.S.; Stewart, A.; Montie, J.E.; Wei, J.T. Prostate carcinoma presentation, diagnosis, and staging: An update form the National Cancer Data Base. Cancer 2003, 98, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 3 1, 578–583. [Google Scholar] [CrossRef]
- Halabi, S.; Vogelzang, N.J.; Kornblith, A.B.; Ou, S.S.; Kantoff, P.W.; Dawson, N.A.; Small, E.J. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, G.; Detti, B.; Scartoni, D.; Lancia, A.; Giacomelli, I.; Baki, M.; Carta, G.; Livi, L.; Santoni, R. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin. Oncol. 2018, 45, 303–315. [Google Scholar] [CrossRef]
- Korporaal, J.G.; van den Berg, C.A.; Jeukens, C.R.; Groenendaal, G.; Moman, M.R.; Luijten, P.; van Vulpen, M.; van der Heide, U.A. Dynamic contrast-enhanced CT for prostate cancer: Relationship between image noise, voxel size, and repeatability. Radiology 2010, 256, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Hussain, M.; Tangen, C.M.; Berry, D.L.; Higano, C.S.; Crawford, E.D.; Liu, G.; Wilding, G.; Prescott, S.; Kanaga Sundaram, S.; Small, E.J.; et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 2013, 368, 1314–1325. [Google Scholar] [CrossRef] [Green Version]
- Perera, M.; Roberts, M.J.; Klotz, L.; Higano, C.S.; Papa, N.; Sengupta, S.; Bolton, D.; Lawrentschuk, N. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat. Rev. Urol. 2020, 17, 469–481. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Heidenreich, A.; Porres, D. Prostate cancer: Treatment sequencing for CRPC—What do we know? Nat. Rev. Urol. 2014, 11, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Shelley, M.; Harrison, C.; Coles, B.; Staffurth, J.; Wilt, T.J.; Mason, M.D. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst. Rev. 2006, 4, CD005247. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciuto, R.; Festa, A.; Rea, S.; Pasqualoni, R.; Bergomi, S.; Petrilli, G.; Maini, C.L. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: A randomized clinical trial. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2002, 43, 79–86. [Google Scholar]
- Hagenbeek, A. Radioimmunotherapy for NHL: Experience of 90Y-ibritumomab tiuxetan in clinical practice. Leuk. Lymphoma 2003, 44 (Suppl. 4), S37–S47. [Google Scholar] [CrossRef]
- Maki, S.; Itoh, Y.; Kubota, S.; Okada, T.; Nakahara, R.; Ito, J.; Kawamura, M.; Naganawa, S.; Yoshino, Y.; Fujita, T.; et al. Clinical outcomes of 125I brachytherapy with and without external-beam radiation therapy for localized prostate cancer: Results from 300 patients at a single institution in Japan. J. Radiat. Res. 2017, 58, 870–880. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- McDermott, R.S.; Greene, J.; McCaffrey, J.; Parker, I.; Helanova, S.; Baird, A.M.; Teiserskiene, A.; Lim, M.; Matthews, H.; Deignan, O.; et al. Radium-223 in combination with enzalutamide in metastatic castration-resistant prostate cancer: A multi-centre, phase II open-label study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211042691. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Jadvar, H.; Ahmadzadehfar, H. PSMA Theranostics: Current Status and Future Directions. Mol. Imaging 2018, 17, 1536012118776068. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Niaz, M.J.; Niaz, M.O.; Tagawa, S.T. Prostate-Specific Membrane Antigen (PSMA)-Targeted Radionuclide Therapies for Prostate Cancer. Curr. Oncol. Rep. 2021, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Kesch, C.; Kretschmer, A.; Tsaur, I.; Ceci, F.; Valerio, M.; Tilki, D.; Marra, G.; Preisser, F.; Fankhauser, C.D.; et al. Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer—A state of the art review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221081922. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Harsini, S.; Saidi, B.; Ahmadzadehfar, H.; Herrmann, K.; Briganti, A.; Walz, J.; Beheshti, M. Factors predicting biochemical response and survival benefits following radioligand therapy with [177Lu]Lu-PSMA in metastatic castrate-resistant prostate cancer: A review. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4028–4041. [Google Scholar] [CrossRef]
- Barna, S.; Haug, A.R.; Hartenbach, M.; Rasul, S.; Grubmüller, B.; Kramer, G.; Blaickner, M. Dose Calculations and Dose-Effect Relationships in 177Lu-PSMA I&T Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Clin. Nucl. Med. 2020, 45, 661–667. [Google Scholar]
- Wu, Y.; Zhang, X.; Zhang, Y.; Xu, B.; Tian, J.; Zhang, J. Optimized 68Ga-Labeled Urea-Based PSMA-Targeted PET Tracers for Prostate Cancer. Pharmaceuticals 2022, 15, 1001. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhou, H.; Xu, B.; Tian, J.; Sun, S.; Zhang, J. Synthesis, preclinical evaluation, and first-in-human study of Al18F-PSMA-Q for prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2774–2785. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhou, H.; Zhang, J. Preclinical development of a novel [68Ga]Ga-/[177Lu]Lu-labeled agent for PSMA-targeted imaging and therapy. J. Radioanal. Nucl. Chem. 2022, 331, 2705–2717. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tombal, B. Non-metastatic CRPC and asymptomatic metastatic CRPC: Which treatment for which patient? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23 (Suppl. 10), x251–x258. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N.; Jr Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Mamawala, M.; Epstein, J.I.; Landis, P.; Wolf, S.; Trock, B.J.; Carter, H.B. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3379–3385. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Zhou, X.C.; Wang, H.; Bassi, S.; Carducci, M.A.; Eisenberger, M.A.; Antonarakis, E.S. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 66, 646–652. [Google Scholar] [CrossRef] [Green Version]
- van Soest, R.J.; de Morrée, E.S.; Kweldam, C.F.; de Ridder, C.; Wiemer, E.; Mathijssen, R.; de Wit, R.; van Weerden, W.M. Targeting the Androgen Receptor Confers In Vivo Cross-resistance Between Enzalutamide and Docetaxel, But Not Cabazitaxel, in Castration-resistant Prostate Cancer. Eur. Urol. 2015, 67, 981–985. [Google Scholar] [CrossRef]
- Gartrell, B.A.; Coleman, R.; Efstathiou, E.; Fizazi, K.; Logothetis, C.J.; Smith, M.R.; Sonpavde, G.; Sartor, O.; Saad, F. Metastatic Prostate Cancer and the Bone: Significance and Therapeutic Options. Eur. Urol. 2015, 68, 850–858. [Google Scholar] [CrossRef]
- Al Nakouzi, N.; Le Moulec, S.; Albigès, L.; Wang, C.; Beuzeboc, P.; Gross-Goupil, M.; de La Motte Rouge, T.; Guillot, A.; Gajda, D.; Massard, C.; et al. Cabazitaxel Remains Active in Patients Progressing After Docetaxel Followed by Novel Androgen Receptor Pathway Targeted Therapies. Eur. Urol. 2015, 68, 228–235. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Heskamp, S.; Konijnenberg, M.; Molkenboer-Kuenen, J.D.; Franssen, G.M.; Clahsen-van Groningen, M.C.; Schottelius, M.; Wester, H.J.; van Weerden, W.M.; Boerman, O.C.; et al. Towards Personalized Treatment of Prostate Cancer: PSMA I&T, a Promising Prostate-Specific Membrane Antigen-Targeted Theranostic Agent. Theranostics 2016, 6, 849–861. [Google Scholar]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 85–90. [Google Scholar]












| [177Lu]Lu-PSMA-BP | [177Lu]Lu-PSMA-4PY | [177Lu]Lu-PSMA-Q | [177Lu]Lu-PSMA-617 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (ID%/g) | SD (ID%/g) | Mean (ID%/g) | SD (ID%/g) | Mean (ID%/g) | SD (ID%/g) | Mean (ID%/g) | SD (ID%/g) | |
| Blood | 0.31 | 0.10 | 0.43 | 0.09 | 0.22 | 0.11 | 0.24 | 0.11 |
| Heart | 0.11 | 0.02 | 0.09 | 0.02 | 0.09 | 0.01 | 0.10 | 0.03 |
| Liver | 0.44 | 0.12 | 0.51 | 0.13 | 0.52 | 0.10 | 0.56 | 0.14 |
| Spleen | 0.40 | 0.09 | 0.42 | 0.14 | 0.48 | 0.14 | 0.78 | 0.22 |
| Lung | 0.38 | 0.08 | 0.29 | 0.08 | 0.29 | 0.09 | 0.33 | 0.10 |
| Kidney | 2.21 | 1.02 | 1.64 | 0.96 | 2.21 | 0.87 | 2.67 | 1.02 |
| Salivary gland | 0.18 | 0.06 | 0.25 | 0.05 | 0.20 | 0.04 | 0.19 | 0.05 |
| Muscle | 0.10 | 0.02 | 0.09 | 0.01 | 0.08 | 0.01 | 0.08 | 0.01 |
| Bone | 0.74 | 0.23 | 0.41 | 0.09 | 0.54 | 0.12 | 0.40 | 0.13 |
| Tumor | 2.98 | 0.21 | 2.61 | 0.49 | 3.87 | 0.32 | 3.74 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, X.; Duan, X.; Yang, X.; Wang, F.; Zhang, J. Optimized Therapeutic 177Lu-Labeled PSMA-Targeted Ligands with Improved Pharmacokinetic Characteristics for Prostate Cancer. Pharmaceuticals 2022, 15, 1530. https://doi.org/10.3390/ph15121530
Wu Y, Zhang X, Duan X, Yang X, Wang F, Zhang J. Optimized Therapeutic 177Lu-Labeled PSMA-Targeted Ligands with Improved Pharmacokinetic Characteristics for Prostate Cancer. Pharmaceuticals. 2022; 15(12):1530. https://doi.org/10.3390/ph15121530
Chicago/Turabian StyleWu, Yitian, Xiaojun Zhang, Xiaojiang Duan, Xing Yang, Feng Wang, and Jinming Zhang. 2022. "Optimized Therapeutic 177Lu-Labeled PSMA-Targeted Ligands with Improved Pharmacokinetic Characteristics for Prostate Cancer" Pharmaceuticals 15, no. 12: 1530. https://doi.org/10.3390/ph15121530
APA StyleWu, Y., Zhang, X., Duan, X., Yang, X., Wang, F., & Zhang, J. (2022). Optimized Therapeutic 177Lu-Labeled PSMA-Targeted Ligands with Improved Pharmacokinetic Characteristics for Prostate Cancer. Pharmaceuticals, 15(12), 1530. https://doi.org/10.3390/ph15121530