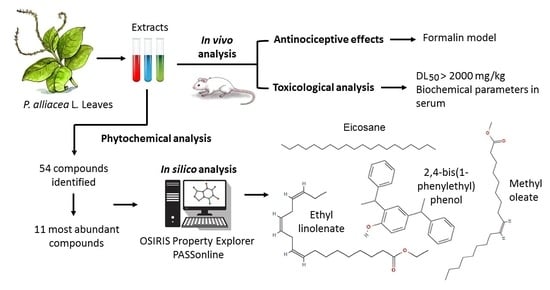
In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves
Abstract
:1. Introduction
2. Results
2.1. Extract Yield
2.2. Evaluation of Antinociceptive Activity
2.3. Toxicity Test
2.4. Identification of Metabolites in P. alliacea L. Leaves
2.4.1. Identification of Components by GC-MS
2.4.2. In Silico Analysis of the Most Abundant Compounds
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Obtaining the Extract
4.3. Extracts and Drugs Used
4.4. Animals
4.5. Evaluation of Antinociceptive Activity
4.6. Toxicity Test
4.7. Identificación de Metabolitos Presentes en el Extracto
4.7.1. Qualitative Analysis by TLC
4.7.2. Quantitative Analysis by Visible Light Spectrophotometry
4.7.3. Identification of Components in Extracts by Gas Chromatography Coupled to Mass Spectrophotometry
4.7.4. In Silico Analysis of the Most Abundant Compounds
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IASP. International Association for the Study of Pain. 2017. Available online: https://www.iasp-pain.org/resources/terminology/#pain (accessed on 14 April 2022).
- Meckes, M.; David-Rivera, A.; Nava-Aguilar, V.; Jiménez, A. Activity of some Mexican medicinal plant extracts on carrageenan-induced rat paw edema. Phytomedicine 2004, 11, 446–451. [Google Scholar] [CrossRef] [PubMed]
- TRAMIL. 2017. Available online: https://www.tramil.net/es/plant/petiveria-alliacea (accessed on 14 April 2022).
- Schroeder, M.A.; Burgos, Á.M. Concentraciones foliares y dinámica estacional de nutrientes en Petiveria alliacea (L.). Rev. Cubana Plant Med. 2011, 16, 374–389. [Google Scholar]
- Rzedowski, J.; de Rzedowski, G.C.; Pátzcuaro, M. Flora Del Bajío Y De Regiones Adyacentes; Centro Regional del Bajío: Pátzcuaro, Mexico, 2000. [Google Scholar]
- Illnait-Ferrer, J. Principales referencias etnomédicas sobre el anamú (Petiveria alliacea linn) y principios activos encontrados en la planta. Un acercamiento al tema. Cienc. Biológicas 2007, 38, 27–30. [Google Scholar]
- Otaiza, R.G.; Arzola, J.C.; Arredondo, M.C.R. Estudio etnobotánico de especies toxicas, ornamentales y medicinales de uso popular, presentes en el Jardín de Plantas Medicinales. Boletín Antropológico 2006, 24, 463–481. [Google Scholar]
- Donado-Orozco, I.; Ruiz-Afanador, T.; Camacho-Romero, O. Estudio etnobotánico piloto de plantas medicinales utilizadas en la zona rural del municipio de Baranoa, Atlántico-Colombia. 2017, pp. 116–117. Available online: https://www.researchgate.net/publication/344956520_Estudio_etnobotanico_piloto_de_plantas_medicinales_utilizadas_en_la_zona_rural_del_municipio_de_Baranoa_Atlantico-Colombia (accessed on 14 April 2022).
- Gomes, P.B.; Oliveira, M.M.d.; Nogueira, C.R.A.; Noronha, E.C.; Carneiro, L.M.V.; Bezerra, J.N.S.; Neto, M.A.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Viana, G.S.B.; et al. Study of Antinociceptive Effect of Isolated Fractions from Petiveria alliacea L. (tipi) in Mice. Biol. Pharm. Bull. 2005, 28, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Martins, R.; Pegoraro, D.; Woisky, R.; Penna, S.; Sertie, J. The anti-inflammatory and analgesic effects of a crude extract of Petiveria alliacea L. (Phytolaccaceae). Phytomedicine 2002, 9, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.O.; Morán, J.M.; Giro, Z.G.; Rodríguez, A.H.; Mujawimana, R.J.; González, K.T.; Frómeta, S.S. In vitro antimicrobial activity of total extracts of the leaves of Petiveria alliacea L. (Anamu). Braz. J. Pharm. Sci. 2013, 49, 241–250. [Google Scholar] [CrossRef]
- Lateef, A.; Folarin, B.I.; Oladejo, S.M.; Akinola, P.O.; Beukes, L.S.; Gueguim-Kana, E.B. Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep. Biochem. Biotechnol. 2018, 48, 646–652. [Google Scholar] [CrossRef]
- García-Pérez, M.E.; Alfonso-Castillo, A.; Lores, O.F.; Batista-Duharte, A.; Lemus-Rodríguez, Z. Toxicological evaluation of an aqueous suspension from leaves and stems of Petiveria alliacea L. (Phytolaccaceae). J. Ethnopharmacol. 2018, 211, 29–37. [Google Scholar] [CrossRef] [Green Version]
- García-González, M.; Coto-Morales, T.; Ocampo, R.; Pazos, L. Subchronic and acute preclinic toxicity and some pharmacological effects of the water extract from leaves of Petiveria alliacea (Phytolaccaceae). Rev. Biol. Trop. 2006, 54, 1323–1326. [Google Scholar] [CrossRef] [Green Version]
- Dickenson, A.H.; Sullivan, A.F. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci. Lett. 1987, 83, 207–211. [Google Scholar] [CrossRef]
- Bannon, A.W.; Malmberg, A.B. Models of Nociception: Hot-Plate, Tail-Flick, and Formalin Tests in Rodents. Curr. Protoc. Neurosci. 2007, 41, 8.9.1–8.9.16. [Google Scholar] [CrossRef]
- Lopéz-Canúl, M. Evaluación del Efecto Anti-Nociceptivo y Anti-Alodinico del Extracto Acuoso de Huichin (Verbesina persicifolia DC) Administrado de Forma Aguda en Ratas Macho de la Cepa Wistar. Julio 2015. Available online: http://cdigital.uv.mx/handle/123456789/42564 (accessed on 15 April 2022).
- Xu, Q.; Wang, Y.; Guo, S.; Shen, Z.; Wang, Y.; Yang, L. Anti-inflammatory and analgesic activity of aqueous extract of Flos populi. J. Ethnopharmacol. 2014, 152, 540–545. [Google Scholar] [CrossRef]
- Díaz-Castillo, M.A. Evaluación de la Actividad Analgésica y Antiinflamatoria de los Extractos Metanólicos de Ormosia coccinea (AubI) Jacks y Macrolobium plttiers (Rose) Scheiy. 2015. Available online: http://up-rid.up.ac.pa/id/eprint/93 (accessed on 16 April 2022).
- Hajhashemi, V.; Ghannadi, A.; Hajiloo, M. Analgesic and Anti-inflammatory Effects of Rosa damascena Hydroalcoholic Extract and its Essential Oil in Animal Models. Iran. J. Pharm. Res. IJPR 2010, 9, 163–168. [Google Scholar]
- Guerra, R.G.L. Analgesic Effect, Phytochemical Characterization and Toxicological Analysis of Ethanolic Extract of Pereskia lychnidiflora Leaves. Rev. Peru. Med. Exp. 2018, 35, 581–590. [Google Scholar]
- Christie, S.L.; Levy, A. Evaluation of the hypoglycaemic activity of Petiveria alliacea (guinea Hen Weed) extracts in normoglycaemic and diabetic rat models. West Indian Med. J. 2013, 62, 685–691. [Google Scholar]
- Oyeleke, A.M.; Adeyemi, O.A.; Egbeyale, E.B.; Obasa, O.A.; Okukenu, O.A. Growth response, organ development and blood indices of growing pullets administered aqueous extracts of Petiveria alliacea. Slovak. J. Anim. Sci. 2020, 69, 310–319. [Google Scholar]
- Muhammad, S.B.; Sobayo, R.A.; Oso, A.O.; Sogunle, O.M.; Ayoola, A.A.; Adeyemo, Y.O.; Basiru, Y.T. Effects of dosage and plant parts of Petiveria alliacea used as phytobiotics on growth, nutrient digestibility and blood profile of Pullet chicks. Arch. Zootec. 2019, 68, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, V. Estudios fenológicos en plantas medicinales VIII. Rev. Cuba. Plantas Med. 1989, 6, 43–52. [Google Scholar]
- De Sous, J.R.; Demuner, A.J.; Pinheiro, J.A.; Breitmaier, E.; Cassels, B.K. Dibenzyl trisulphide and transN-methyl-a-methoxyproline from Petiveria alliacea. Phytochemistry 1990, 29, 3653–3655. [Google Scholar] [CrossRef]
- Benevides PJ, C.; Young MC, M.; Giesbrecht, A.M.; Roque, N.F.; da SBolzani, V. Antifungal polysulphides from Petiveria alliacea L. Phytochemistry 2001, 57, 743–747. [Google Scholar] [CrossRef]
- Haggerty, B.M. The Phenology Handbook. A Guide to Phenological Monitoring for Students, Teachers, Families, and Nature Enthusiasts. University of California: Santa Barbara, CA, USA, 2008; Available online: https://www.usanpn.org/files/shared/files/Haggerty&Mazer_ThePhenologyHandbook_v3Aug2009.pdf (accessed on 16 April 2022).
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef]
- Santos, C.C.d.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; Santos, R.N.d. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.C.; Martins BP, M.P.; Medeiros, D.L.; Santos, S.V.; Gayer, C.R.; Velozo, L.S.; Coelho, M.G. Antinociceptive and anti-inflammatory activities of the hexanic extract of Echinodorus macrophyllus (Kunth) Micheli in mice. Braz. J. Health Biomed. Sci. 2019, 18, 25–32. [Google Scholar]
- Tamoto, K.; Yamazaki, A.; Nochi, H.; Miura, T. Ozonides of olive oil and methyl oleate inhibit the expression of cyclooxygense-2 through the suppression of IkB/NFkB-dependent pathway in lipopolysaccharide-stimulated macrophage-like THP-1 cells. In Proceedings of the IOA 17th World Ozone Crongress, Strasbourg, France, 22–25 August 2005; pp. 1–6. [Google Scholar]
- Hossain, M.A.; Al-Toubi, W.A.; Weli, A.M.; Al-Riyami, Q.A.; Al-Sabahi, J.N. Hossain. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J. Taibah Univ. Sci. 2013, 7, 181–188. [Google Scholar] [CrossRef]
- Soares, D.G.; Andreazza, A.A.C.; Salvador, M. Sequestering Ability of Butylated Hydroxytoluene, Propyl Gallate, Resveratrol, and Vitamins C and E against ABTS, DPPH, and Hydroxyl Free Radicals in Chemical and Biological DPPH, and Hydroxyl Free Radicals in Chemical and Biological Systems. J. Agric. Food Chem. 2013, 51, 1077–1080. [Google Scholar] [CrossRef]
- Gao, X.; Kim, H.K.; Chung, J.M.; Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007, 131, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Hacimuftuoglu, A.; Handy, C.R.; Goettl, V.M.; Lin, C.G.; Dane, S.; Stephens, R.L. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav. Brain Res. 2006, 173, 211–216. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Guerrero, R.V.; Vargas, R.A.; Petricevich, V.L. Chemical Compounds and Biological Activity of an Extract from Bougainvillea x Buttiana (var. Rose) Holttum and Standl. Int. J. Pharm. Pharm. Sci. 2017, 9, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Pratiwi, P.; Fathoni, A.; Efendi, O.; Agusta, A. Antioxidant, Antibacterial Activity and GC-MS Analysis of Extract of Giant Forest ANT Dinomyrmex gigas. J. Biodjati 2019, 4, 263–277. [Google Scholar]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current Insights into the Biological Action of Squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmad, S.; Rao, H.; Shaukat, U.; Shahzad, M.N.; Sajid-ur-Rehman, M.; Basit, A.; Arshad, M.A.; Ahmad, B. Multi-Method Determination of Antioxidant Capacity, Phytochemical and Biological Investigation of Four Different Solvent Extractives of Leucophyllum frutescens (cenizo). S. Afr. J. Bot. 2020, 148, 200–209. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Hamid, S.B.A.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- International Labour Organization. Butylated Hydroxytoluene. March 1999. Available online: https://www.ilo.org/dyn/icsc/showcard.display?p_card_id=0841&p_version=2&p_lang=en (accessed on 17 April 2022).
- PubChem. Bis(2-Ethylhexyl) Maleate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bis_2-ethylhexyl_-maleate#section=Safety-and-Hazards (accessed on 17 April 2022).
- Zimmermann, M. Pautas éticas para investigaciones de dolor experimental en animales conscientes. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- NOM. Norma Oficial Mexicana. NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado. NOM-062-ZOO-1999. Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 22 March 2022).
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. Review Article The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2008; p. 27. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography; Springer-Verlag: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Robertson, A.H.M. A critical investigation into the flavognost Method for Thea flavin Analysis in Black Tea. Food Chem. 1989, 34, 57–70. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sim, E.W.; Lai, S.Y.; Chang, Y.P. Antioxidant capacity, nutritional and phytochemical content of peanut (Arachis hypogaea L.) shells and roots. Afr. J. Biotechnol. 2012, 11, 11547–11551. [Google Scholar]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-rizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J. Pharm. Sci. 2008, 32, 17–20. [Google Scholar]
- EPA. Method 8270D Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). 2014. Available online: https://archive.epa.gov/epa/sites/production/files/2015-12/documents/8270d.pdf (accessed on 16 March 2022).
- Sander, T. OSIRIS Property Explorer. 2013. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 18 April 2022).


| Biochemical Indicator | Control Group | Metanoica Extract | Hexanic Extract | Aqueous Extract | Literature Range |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 127.6 ± 22.61 | 172.60 ± 31.47 * | 113 ± 22.75 | 170.33 ± 10.02 * | 104–290.3 |
| Cholesterol (mg/dL) | 130 ± 22.46 | 107.20 ± 24.34 | 105.60 ± 21.44 | 123.33 ± 2.08 | 67–119.69 |
| Triglyceride (mg/dL) | 173.20 ± 41.14 | 95.20 ± 25.57 ** | 149.8 ± 41.55 | 73.00 ± 12.12 *** | 54–197.35 |
| Albumin (g/dL) | 4.98 ± 0.67 | 4.60 ± 0.43 | 4.94 ± 0.28 | 4.27 ± 0.31 | 2.1–4.04 |
| Total protein (g/dL) | 6.22 ± 0.48 | 4.66 ± 0.22 *** | 6.12 ± 0.28 | 5.90 ± 0.17 | 4–6 |
| Uric acid (mg/dL) | 7.38 ± 1.05 | 5.00 ± 2.04 * | 6.12 ± 1.06 | 1.78 ± 0.52 *** | 2.06–3.22 |
| Amylase (U/L) | 1336 ± 89.23 | 806.67 ± 233.21 * | 1118.5 ± 88.39 | 1324.40 ± 121.01 | 607.6–2756 |
| Secondary Metabolites | Methanolic Extract | Hexanic Extract | Aqueous Extract |
|---|---|---|---|
| Saponins (mg diosgenin/mL) | 1.65 ± 0.54 | - | 0.40 ± 0.02 *** |
| Total flavonoids (mg rutin eq/mL) | 0.52 ± 0.06 | 0.02 ± 0.00 *** | 0.15 ± 0.01 ** |
| Flavones and flavonols (mg quercetin eq/mL) | 0.71 ± 0.005 | 0.008 ± 0.00 *** | 0.124 ± 0.03 ** |
| Total Phenols (mg gallic acid/mL) | 0.20 ± 0.02 | 0.029 ± 0.00 ** | 0.55 ± 0.01 *** |
| Terpenes (mg ursolic acid/mL) | 0.62 ± 0.01 | 0.55 ± 0.04 * | 0.231 ± 0.02 *** |
| Coumarins (mg umberylferone/mL) | 0.142 ± 0.02 | 0.044 ± 0.01 *** | 0.090 ±0.01 * |
| Extract | R.T. (min) | Name | A% | Class |
|---|---|---|---|---|
| Methanolic | 8.812 | Ethyl palmitate | 4.32 | Fatty ester |
| 9.813 | Phytol | 48.80 | Terpene | |
| 9.974 | Ethyl linolenate | 17.84 | Fatty ester | |
| 13.501 | Squalen | 7.24 | Terpene | |
| Hexanic | 5.298 | Butylated hydroxytoluene | 6.04 | Phenolic compound |
| 9.552 | Methyl oleate | 12.93 | Fatty ester | |
| 11.815 | Eicosane | 6.25 | Hydrocarbon | |
| 13.501 | Squalen | 10.29 | Terpene | |
| Aqueous | 8.363 | Methyl 14-methylpentadecanoate | 7.0 | Fatty ester |
| 9.543 | Methyl oleate | 14.51 | Fatty ester | |
| 9.851 | Bis(2-ethylhexyl) maleate | 36.27 | Ester | |
| 10.195 | Octadecyl acetate | 1.49 | Fatty ester | |
| 12.106 | 2,4-Bis(1-phenylethyl)phenol | 3.03 | Phenolic compound |
| Extract | Compounds | Antinociceptive | Antiinflammatory | ||
|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | ||
| Methanolic | Ethyl palmitate | 0.472 | 0.054 | 0.600 | 0.032 |
| Methanolic | Phytol | 0.300 | 0.182 | 0.458 | 0.070 |
| Methanolic | Ethyl linolenate | 0.509 | 0.031 | 0.827 | 0.005 |
| Methanolic/ Hexanic | Squalene | 0.474 | 0.053 | 0.701 | 0.016 |
| Hexanic | Butylated hidroxytoluene | 0.498 | 0.037 | 0.803 | 0.006 |
| Hexanic Aqueous | Methyl oleate | 0.573 | 0.011 | 0.607 | 0.030 |
| Hexanic | Eicosane | 0.595 | 0.012 | 0.424 | 0.004 |
| Aqueous | Methyl 14-methylpentadecanoate | 0.490 | 0.042 | 0.392 | 0.1 |
| Aqueous | Bis(2-ethylhexyl) maleate | 0.331 | 0.160 | 0.605 | 0.030 |
| Aqueous | Octadecyl acetate | 0.455 | 0.067 | 0.717 | 0.014 |
| Aqueous | 2,4-bis(1-phenylethyl) phenol | 0.555 | 0.014 | 0.318 | 0.145 |
| Extract | Compounds | M. | T. | I. | R.E. |
|---|---|---|---|---|---|
| Methanolic | Ethyl palmitate |  |  |  |  |
| Methanolic | Phytol |  |  |  |  |
| Methanolic | Ethyl linolenate |  |  |  |  |
| Methanolic/Hexanic | Squalene |  |  |  |  |
| Hexanic | Butylated hidroxytoluene |  |  |  |  |
| Hexanic Aqueous | Methyl oleate |  |  |  |  |
| Hexanic | Eicosane |  |  |  |  |
| Aqueous | Methyl 14-methylpentadecanoate |  |  |  |  |
| Aqueous | Bis(2-ethylhexyl) maleate |  |  |  |  |
| Aqueous | Octadecyl acetate |  |  |  |  |
| Aqueous | 2,4-bis(1-phenylethyl) phenol |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Salomón, K.d.C.; Cruz-Rodríguez, R.I.; Espinosa-Juárez, J.V.; Cruz-Salomón, A.; Briones-Aranda, A.; Ruiz-Lau, N.; Ruíz-Valdiviezo, V.M. In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves. Pharmaceuticals 2022, 15, 943. https://doi.org/10.3390/ph15080943
Cruz-Salomón KdC, Cruz-Rodríguez RI, Espinosa-Juárez JV, Cruz-Salomón A, Briones-Aranda A, Ruiz-Lau N, Ruíz-Valdiviezo VM. In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves. Pharmaceuticals. 2022; 15(8):943. https://doi.org/10.3390/ph15080943
Chicago/Turabian StyleCruz-Salomón, Kelly del Carmen, Rosa Isela Cruz-Rodríguez, Josué Vidal Espinosa-Juárez, Abumalé Cruz-Salomón, Alfredo Briones-Aranda, Nancy Ruiz-Lau, and Víctor Manuel Ruíz-Valdiviezo. 2022. "In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves" Pharmaceuticals 15, no. 8: 943. https://doi.org/10.3390/ph15080943
APA StyleCruz-Salomón, K. d. C., Cruz-Rodríguez, R. I., Espinosa-Juárez, J. V., Cruz-Salomón, A., Briones-Aranda, A., Ruiz-Lau, N., & Ruíz-Valdiviezo, V. M. (2022). In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves. Pharmaceuticals, 15(8), 943. https://doi.org/10.3390/ph15080943