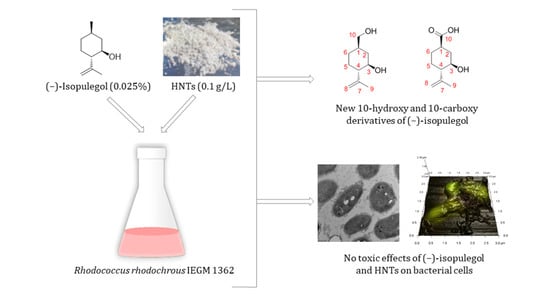
Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening for a Target Rhodococcus Strain and Evaluation of Its Response to (–)-Isopulegol and/or HNTs
2.2. Biotransformation of (–)-Isopulegol by R. rhodochrous IEGM 1362: The State and Activity of Cells
2.3. Identification of Products of (–)-Isopulegol Biotransformation
2.4. In Silico Analysis of (–)-Isopulegol and Its Derivatives
3. Materials and Methods
3.1. Microorganisms
3.2. Reagents and Nanomaterials
3.3. Cultivation Conditions
3.4. Phase-Contrast and Fluorescent Microscopy
3.5. Atomic Force and Confocal Laser Scanning Microscopy (AFM-CLSM)
3.6. Transmission Electron Microscopy (TEM)
3.7. Energy Dispersive X-ray Spectroscopy (EDX) with Elemental Mapping
3.8. Zeta Potential Measurement
3.9. Metabolic Activity Measurements
3.10. Extraction and Analysis of Residual (–)-Isopulegol and Its Derivatives
3.11. X-ray Data for Compounds 2 and 3
3.12. In Silico Analysis of (–)-Isopulegol and Its Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| CCDC | the Cambridge Crystallographic Data Center |
| CLSM | confocal laser scanning microscopy |
| EDX | energy dispersive X-ray spectrometry |
| GC-MS | gas chromatography-mass spectrometry |
| TEM | transmission electron microscopy |
| TLC | thin layer chromatography |
| HMBC | heteronuclear multiple bond correlation |
| HNTs | halloysite nanotubes |
| HR-MS | high resolution mass spectrometry |
| NMR | nuclear magnetic resonance |
| OD | optical density |
References
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91, e20190105. [Google Scholar] [CrossRef]
- Sultana, N.; Saify, Z.S. Enzymatic biotransformation of terpenes as bioactive agents. J. Enzyme Inhib. Med. Chem. 2013, 28, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Zaprutko, L. Anti-COVID drugs: Repurposing existing drugs or search for new complex entities, strategies and perspectives. Future Med. Chem. 2020, 12, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Minh Le, T.; Szakonyi, Z. Enantiomeric isopulegol as the chiral pool in the total synthesis of bioactive agents. Chem. Rec. 2022, 22, e202100194. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on isopulegol. Food Chem. Toxicol. 2008, 46, S185–S189. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, I.V.; Zarubaev, V.V.; Lavrentieva, I.N.; Shtro, A.A.; Esaulkova, I.L.; Korchagina, D.V.; Borisevich, S.S.; Volcho, K.P.; Salakhutdinov, N.F. Highly potent activity of isopulegol-derived substituted octahydro-2H-chromen-4-ols against influenza A and B viruses. Bioorg. Med. Chem. Lett. 2018, 28, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Li-Zhulanov, N.S.; Pavlova, A.V.; Korchagina, D.V.; Gatilov, Y.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis of 1,3-oxazine derivatives based on (−)-isopulegol using the Ritter reaction and study of their analgesic activity. Chem. Heterocycl. Compd. 2020, 56, 936–941. [Google Scholar] [CrossRef]
- Le, T.M.; Huynh, T.; Endre, G.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds. RSC Adv. 2020, 10, 38468–38477. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Synthesis and transformation of (−)-isopulegol-based chiral β-aminolactones and β-aminoamides. Int. J. Mol. Sci. 2018, 19, 3522. [Google Scholar] [CrossRef] [PubMed]
- Kholkina, E.; Mäki-Arvela, P.; Lozachmeuer, C.; Barakov, R.; Shcherban, N.; Murzin, D.Y. Prins cyclisation of (−)-isopulegol with benzaldehyde over ZSM-5 based micro-mesoporous catalysts for production of pharmaceuticals. Chin. J. Catal. 2019, 40, 1713–1720. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ruhoy, I.S. Green pharmacy and pharmEcovigilance: Prescribing and the planet. Expert Rev. Clin. Pharmacol. 2011, 4, 211–232. [Google Scholar] [CrossRef]
- Kümmerer, K. Why green and sustainable pharmacy? In Green and Sustainable Pharmacy; Kümmerer, K., Hempel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–11. ISBN 9783642051982. [Google Scholar]
- Leder, C.; Rastogi, T.; Kümmerer, K. Putting benign by design into practice-novel concepts for green and sustainable pharmacy: Designing green drug derivatives by non-targeted synthesis and screening for biodegradability. Sustain. Chem. Pharm. 2015, 2, 31–36. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.E.H.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial biotransformation as a tool for drug development based on natural products from mevalonic acid pathway: A review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Su, A.; Kiokekli, S.; Naviwala, M.; Shirke, A.N.; Pavlidis, I.V.; Gross, R.A. Cutinases as stereoselective catalysts: Specific activity and enantioselectivity of cutinases and lipases for menthol and its analogs. Enzyme Microb. Technol. 2020, 133, 109467. [Google Scholar] [CrossRef]
- Garzón-Posse, F.; Becerra-Figueroa, L.; Hernández-Arias, J.; Gamba-Sánchez, D. Whole cells as biocatalysts in organic transformations. Molecules 2018, 23, 1265. [Google Scholar] [CrossRef]
- Anteneh, Y.S.; Franco, C.M.M. Whole cell actinobacteria as biocatalysts. Front. Microbiol. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Busch, H.; Hagedoorn, P.L.; Hanefeld, U. Rhodococcus as a versatile biocatalyst in organic synthesis. Int. J. Mol. Sci. 2019, 20, 4787. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.V.; Grishko, V.V.; Ivshina, I.B. Cell adaptations of Rhodococcus rhodochrous IEGM 66 to betulin biotransformation. Process. Biochem. 2017, 52, 1–9. [Google Scholar] [CrossRef]
- Cheremnykh, K.M.; Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Bioconversion of ecotoxic dehydroabietic acid using Rhodococcus actinobacteria. J. Hazard. Mater. 2018, 346, 103–112. [Google Scholar] [CrossRef]
- Shukla, O.P.; Bartholomus, R.C.; Gunsalus, I.C. Microbial transformation of menthol and menthane-3,4-diol. Can. J. Microbiol. 1987, 33, 489–497. [Google Scholar] [CrossRef]
- Shahcheraghi, N.; Golchin, H.; Sadri, Z.; Tabari, Y.; Borhanifar, F.; Makani, S. Nano-biotechnology, an applicable approach for sustainable future. 3 Biotech. 2022, 12, 65. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Hasan, S.K.; Zainuddin, S.; Tanthongsack, J.; Hosur, M.V.; Allen, L. A study of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) biofilms’ thermal and biodegradable properties reinforced with halloysite nanotubes. J. Compos. Mater. 2018, 52, 3199–3207. [Google Scholar] [CrossRef]
- Ahn, H.; Rehman, J.U.; Kim, T.; Oh, M.S.; Yoon, H.Y.; Kim, C.; Lee, Y.; Shin, S.G.; Jeon, J.R. Fungal mycelia functionalization with halloysite nanotubes for hyphal spreading and sorption behavior regulation: A new bio-ceramic hybrid for enhanced water treatment. Water Res. 2020, 186, 116380. [Google Scholar] [CrossRef]
- Partovinia, A.; Koosha, M. Fabrication of novel nanocomposite nanofibrous matrices retaining high concentration of microbial cells for heavy crude oil biodegradation. Express Polym. Lett. 2019, 13, 484–499. [Google Scholar] [CrossRef]
- Tian, D.; Shen, F.; Hu, J.; Renneckar, S.; Saddler, J.N. Enhancing bacterial cellulose production via adding mesoporous halloysite nanotubes in the culture medium. Carbohydr. Polym. 2018, 198, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, P.; Wang, F.; Niu, W.; Ahmed, Z.; Chen, M.; Lu, G.; Dang, Z. Differential regulation and the underlying mechanisms of clay minerals to Escherichia coli under the stress of polymyxin B: Comparing halloysite with kaolinite. Chemosphere 2021, 265, 129095. [Google Scholar] [CrossRef]
- Prinz Setter, O.; Movsowitz, A.; Goldberg, S.; Segal, E. Antibody-functionalized halloysite nanotubes for targeting bacterial cells. ACS Appl. Bio Mater. 2021, 4, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Mulyukin, A.L.; Sorokin, V.V.; Loiko, N.G.; Suzina, N.E.; Duda, V.I.; Vorob’eva, E.A.; El’-Registan, G.I. Comparative study of the elemental composition of vegetative and dormant microbial cells. Microbiology 2002, 71, 31–40. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Glebov, G.G.; Ivshina, I.B. Effects of nickel nanoparticles on Rhodococcus cell surface morphology and nanomechanical properties. Nanomaterials 2022, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Mirata, M.A.; Holtmann, D.; Schrader, J. Biooxidation of monoterpenes with bacterial monooxygenases. Process. Biochem. 2011, 46, 1885–1899. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of nanoclays on the biodegradation of poly(lactic acid) nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef]
- Fernandes, P.; Cabral, J.M.S. Microlitre/millilitre shaken bioreactors in fermentative and biotransformation processes—A review. Biocatal. Biotransformat. 2006, 24, 237–252. [Google Scholar] [CrossRef]
- Sassa, T.; Kenmoku, H.; Sato, M.; Murayama, T.; Kato, N. (+)-Menthol and its hydroxy derivatives, novel fungal monoterpenols from the fusicoccin-producing fungi, Phomopsis amygdali F6a and Niigata 2. Biosci. Biotechnol. Biochem. 2003, 67, 475–479. [Google Scholar] [CrossRef]
- Asakawa, Y.; Takahashi, H.; Toyota, M.; Noma, Y. Biotransformation of monoterpenoids, (−)- and (+)-Menthols, terpinolene and carvotanacetone by Aspergillus species. Phytochemistry 1991, 30, 3981–3987. [Google Scholar] [CrossRef]
- Sawamura, N.; Shima, S.; Sakai, H. Conversion of (−)-menthone by Pseudomonas fluorescens M-2. Agric. Biol. Chem. 1976, 40, 649–653. [Google Scholar] [CrossRef]
- Catalogue of the Strains of Regional Specialised Collection of Alkanotrophic Microorganisms. Available online: http://www.iegmcol.ru/strains/index.html (accessed on 6 April 2022).
- Postgate, J.R. Differential media for sulphur bacteria. J. Sci. Food Agric. 1959, 10, 669–674. [Google Scholar] [CrossRef]
- Mrunalini, B.R.; Girisha, S.T. Screening and characterization of lipid inclusions in bacteria by fluorescence microscopy and mass spectrometry as a source for biofuel production. Indian J. Sci. Technol. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Way2Drug Predictive Services. PASS Online. Available online: http://www.pharmaexpert.ru/passonline/index.php (accessed on 6 April 2022).





 ), (‒)-isopulegol (
), (‒)-isopulegol (  ), and HNTs (
), and HNTs (  ). Biotic control (
). Biotic control (  ), abiotic control (
), abiotic control (  ).
).
 ), (‒)-isopulegol (
), (‒)-isopulegol (  ), and HNTs (
), and HNTs (  ). Biotic control (
). Biotic control (  ), abiotic control (
), abiotic control (  ).
).






| Culture Conditions | Days | (–)-Isopulegol (m/z 154, 7.84 min) | Compound 2 (m/z 170, 10.66 min) | Compound 3 (m/z 184, 11.50 min) |
|---|---|---|---|---|
| Medium, 25 mL | ||||
| (–)-Isopulegol | 2 | 15.2 | 84.8 | − |
| 3 | 45.7 | − | 54.3 | |
| 5 | 19.4 | − | 80.6 | |
| (–)-Isopulegol and HNTs | 2 | 40.8 | 59.2 | − |
| 3 | − | 100 | − | |
| 5 | 12.0 | 72.2 | 15.7 | |
| Medium, 100 mL | ||||
| (–)-Isopulegol | 2 | − | − | 100 |
| (–)-Isopulegol and HNTs | 2 | − | − | 100 |
| Test Organisms (Index, Exposure) | Concentration, mg/L | ||
|---|---|---|---|
| (–)-Isopulegol | Compound 2 | Compound 3 | |
| Water solubility at 25 °C | 308.6 | 345.2 | 6303.00 |
| ECOSAR Class | Neutral Organics | Neutral Organics | Neutral Organics-acid |
| Acute toxicity | |||
| Fish (LD50, 96 h) | 7.39 | 72.75 | 1138.44 |
| Daphnia (LD50, 48 h) | 4.76 | 42.53 | 654.68 |
| Green algae (ED50, 96 h) | 5.99 | 35.74 | 513.99 |
| Chronic toxicity | |||
| Fish (ED50, 30 d) | 0.84 | 7.36 | 112.96 |
| Daphnia (ED50, 21 d) | 0.66 | 4.49 | 66.15 |
| Green algae (ED50, 16 d) | 2.08 | 9.98 | 138.47 |
| Estimated Activity | (–)-Isopulegol | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | |
| Carminative | 0.976 | 0.000 | 0.930 | 0.001 | 0.928 | 0.001 |
| Anti–eczema | 0.929 | 0.004 | 0.918 | 0.004 | 0.908 | 0.004 |
| Neuromuscular acetyl choline blocker | 0.751 | 0.003 | 0.677 | 0.005 | 0.682 | 0.005 |
| Inhibitor of β-glucuronidase | – | – | 0.670 | 0.005 | 0.714 | 0.004 |
| Immunosuppressive | 0.755 | 0.010 | 0.720 | 0.014 | 0.731 | 0.013 |
| Stimulator of NFκB transcription factor | 0.747 | 0.003 | 0.730 | 0.004 | 0.730 | 0.004 |
| Inhibitor of retinol dehydrogenase | 0.738 | 0.002 | 0.739 | 0.002 | 0.693 | 0.002 |
| Respiratory analeptic | – | – | 0.568 | 0.026 | 0.686 | 0.016 |
| Anti-inflammatory | 0.690 | 0.024 | 0.645 | 0.024 | 0.692 | 0.013 |
| Antitumor | – | – | 0.550 | 0.057 | 0.482 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivshina, I.B.; Luchnikova, N.A.; Maltseva, P.Y.; Ilyina, I.V.; Volcho, K.P.; Gatilov, Y.V.; Korchagina, D.V.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L.; et al. Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous. Pharmaceuticals 2022, 15, 964. https://doi.org/10.3390/ph15080964
Ivshina IB, Luchnikova NA, Maltseva PY, Ilyina IV, Volcho KP, Gatilov YV, Korchagina DV, Kostrikina NA, Sorokin VV, Mulyukin AL, et al. Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous. Pharmaceuticals. 2022; 15(8):964. https://doi.org/10.3390/ph15080964
Chicago/Turabian StyleIvshina, Irina B., Natalia A. Luchnikova, Polina Yu. Maltseva, Irina V. Ilyina, Konstantin P. Volcho, Yurii V. Gatilov, Dina V. Korchagina, Nadezhda A. Kostrikina, Vladimir V. Sorokin, Andrey L. Mulyukin, and et al. 2022. "Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous" Pharmaceuticals 15, no. 8: 964. https://doi.org/10.3390/ph15080964
APA StyleIvshina, I. B., Luchnikova, N. A., Maltseva, P. Y., Ilyina, I. V., Volcho, K. P., Gatilov, Y. V., Korchagina, D. V., Kostrikina, N. A., Sorokin, V. V., Mulyukin, A. L., & Salakhutdinov, N. F. (2022). Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous. Pharmaceuticals, 15(8), 964. https://doi.org/10.3390/ph15080964