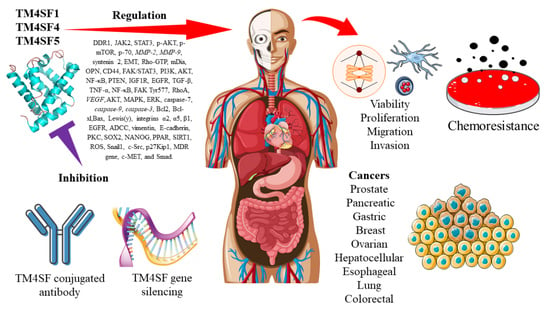
Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance
Abstract
:1. Introduction
2. Transmembrane 4 Superfamily
3. The Regulatory Roles of TM4SF1 in Different Cancer Types
3.1. Prostate Cancer
3.2. Pancreatic Cancer
3.3. Gastric Cancer
3.4. Breast Cancer
3.5. Ovarian Cancer
3.6. Hepatocellular Carcinoma
3.7. Bladder Cancer
4. Molecular Expression, Regulatory Roles, Mechanisms, and Biomolecular Interactions of TM4SF4 in Different Cancers
4.1. Lung Cancer
4.2. Colorectal Cancer
4.3. Hepatocellular Carcinoma
5. Molecular Expression, Regulatory Roles, Mechanisms, and Biomolecular Interactions of TM4SF5 in Different Cancers
5.1. Hepatocellular Carcinoma
5.2. Esophageal Cancer
5.3. Pancreatic Cancer, Colorectal Cancer, and Gastric Cancer
6. The Regulatory Roles and Molecular Mechanisms of TM4SF1, TM4SF4, and TM4SF5 in Cancer Chemoresistance
7. The Current Use of Antibodies in Targeting TM4SF as a Potential Cancer Treatment
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. In Metastatic Cancer: Clinical and Biological Perspectives; Jandial Rahul, Ed.; Landes Bioscience: Austin, TX, USA, 2013; pp. 2000–2013. [Google Scholar]
- She, X.Y.; Gao, Y.; Zhao, Y.; Yin, A.; Dong, Z. A high-throughput screen identifies inhibitors of lung cancer stem cells. Biomed. Pharmacother. 2021, 140, 111748. [Google Scholar] [CrossRef] [PubMed]
- Golemis, E.A.; Scheet, P.; Beck, T.; Scolnick, E.; Hunter, D.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Zhang, Q.; Yu, X.; He, Y.; Guo, W. FENDRR: A pivotal, cancer-related, long non-coding RNA. Biomed. Pharmacother. 2021, 137, 111390. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Mittal, V.; Ban, Y.; Lourenco, A.; Yomtoubian, S.; Lee, S. Metastatic tumor cells-genotypes and phenotypes. Front. Biol. 2018, 13, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [PubMed]
- Sommerová, L.; Ondroušková, E. HR Cancer Cells as Dynamic System—Molecular and Phenotypic Changes During Tumor Formation, Progression and Dissemination. Klin. Onkol. 2016, 29, 6–11. [Google Scholar] [CrossRef]
- Wu, Y.S.; Looi, C.; Subramaniam, K.; Masamune, I.; Chung, I. Soluble factors from stellate cells induce pancreatic cancer cell proliferation via Nrf2-activated metabolic reprogramming and ROS detoxification. Oncotarget 2016, 7, 36719–36732. [Google Scholar] [CrossRef] [Green Version]
- Ramli, S.; Sim, M.; Guad, R.; Gopinath, S.; Subramaniyan, V.; Fuloria, S.; Fuloria, N.; Choy, K.; Rana, S.; Wu, Y. Long Noncoding RNA UCA1 in Gastrointestinal Cancers: Molecular Regulatory Roles and Patterns, Mechanisms, and Interactions. J. Oncol. 2021, 2021, 5519720. [Google Scholar] [CrossRef]
- Belete, T.M. Recent Updates on the Development of Deuterium-Containing Drugs for the Treatment of Cancer. Drug Des. Dev. Ther. 2022, 16, 3465–3472. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- WHO. Cancer. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 August 2019).
- Gao, C.; Yao, H.; Liu, H.; Feng, Y.; Yang, Z. TM4SF1 is a potential target for anti-invasion and metastasis in ovarian cancer. BMC Cancer 2019, 19, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.M.; Park, S.; Kim, S.; Kim, H.; Lee, B.; Kim, J.; Park, J.; Kim, S.; Yang, H.; Kim, W.; et al. KIAA1324 Suppresses Gastric Cancer Progression by Inhibiting the Oncoprotein GRP78. Cancer Res. 2015, 75, 3087–3097. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Pu, C.; Tang, J.; Wang, Y.; Wang, C.; Qiu, Z.; Xiang, T.; Zhang, Y.; Peng, W. Transmembrane-4 L-six family member-1 (TM4SF1) promotes non-small cell lung cancer proliferation, invasion and chemo-resistance through regulating the DDR1/Akt/ERK-mTOR axis. Respir Res. 2019, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, M.; Qi, Y.; Wang, H.; Liu, M.; Liu, Q.; Lin, B. Lewis(y) antigen-mediated positive feedback loop induces and promotes chemotherapeutic resistance in ovarian cancer. Int. J. Oncol. 2018, 53, 1774–1786. [Google Scholar] [CrossRef]
- Druker, B.J.; Sawyers, C.; Kantarjian, H.; Resta, D.; Reese, S.; Ford, J.; Capdeville, R.; Talpaz, M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 2001, 344, 1038–1042. [Google Scholar] [CrossRef] [Green Version]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.; Verduzco, D.; Bashir, A.; Mohammed, O.; Elhassan, G.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-g. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Treude, F.; Krämer, O.; Lüscher, B.; Hartkamp, J. PAR-4 overcomes chemo-resistance in breast cancer cells by antagonizing cIAP1. Sci. Rep. 2019, 9, 8755. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yu, X.; Zheng, Q.; Zhang, Q.; He, Y.; Guo, W. Promising role of long non-coding RNA PCAT6 in malignancies. Biomed. Pharmacother. 2021, 137, 111402. [Google Scholar] [CrossRef]
- Cai, S.; Deng, Y.; Peng, H.; Shen, J. Role of Tetraspanins in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 723341. [Google Scholar] [CrossRef]
- Ahn, H.M.; Ryu, J.; Song, J.; Lee, Y.; Kim, H.; Ko, D.; Choi, I.; Kim, S.; Lee, J.; Kim, S. Anti-cancer Activity of Novel TM4SF5-Targeting Antibodies through TM4SF5 Neutralization and Immune Cell-Mediated Cytotoxicity. Theranostics 2017, 7, 594–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Tang, H.; Wu, J.; Gao, T.; Li, J. Tspan5 inhibits proliferation and migration of JEG-3 cells by inhibiting FAK and AKT phosphorylation. Obstet. Gynecol. 2019, 46, 280–286. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, S.; Trojanowicz, B.; Liu, N.; Zhu, G.; Dralle, H.; Hoang-Vu, C. Down-regulation of TM4SF is associated with the metastatic potential of gastric carcinoma TM4SF members in gastric carcinoma. World J. Surg. Oncol. 2011, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, M.D.; Tomlinson, M.G. The ins and outs of the transmembrane 4 superfamily. Immunol Today 1994, 15, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Allioli, N.; Vincent, S.; Vlaeminck-Guillem, V.; Decaussin-Petrucci, M.; Ragage, F.; Ruffion, A.; Samarut, J. TM4SF1, a novel primary androgen receptor target gene over-expressed in human prostate cancer and involved in cell migration. Prostate 2011, 71, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.D.; Ni, J.; Rudy, G. The L6 membrane proteins—A new four-transmembrane superfamily. Protein. Sci. 2000, 9, 1594–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.; Lee, J.W. TM4SF5-Mediated Roles in the Development of Fibrotic Phenotypes. Mediat. Inflamm. 2017, 2017, 5108525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Ohuchida, K.; Cui, L.; Zhao, M.; Shindo, K.; Fujiwara, K.; Manabe, T.; Torata, N.; Moriyama, T.; Miyasaka, Y.; et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int. J. Oncol. 2015, 47, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Yang, J.; Ramachandran, V.; Arumugam, T.; Deng, D.; Li, Z.; Xu, L.; Logsdon, C. TM4SF1 Regulates Pancreatic Cancer Migration and Invasion In Vitro and In Vivo. Cell Physiol. Biochem. 2016, 39, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y. Overexpression of S100B, TM4SF4, and OLFM4 genes is correlated with liver metastasis in Taiwanese colorectal cancer patients. DNA Cell Biol. 2012, 31, 43–49. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, M.; Zhao, Z.; Huang, H.; Fu, W.; Xu, X. Expression of Tspan-1 gene in patients with advanced gastric cancer. Oncol. Lett. 2017, 14, 2996–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, H.P.; Frank, I.; Berry, K.; Senter, P. Therapeutic effects of monoclonal antibody-beta-lactamase conjugates in combination with a nitrogen mustard anticancer prodrug in models of human renal cell carcinoma. J. Med. Chem. 1998, 41, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Wice, B.M.; Gordon, J.I. A tetraspan membrane glycoprotein produced in the human intestinal epithelium and liver that can regulate cell density-dependent proliferation. J. Biol. Chem. 1995, 270, 21907–21918. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, M.; Yokoyama, K.; Li, T. Molecular cloning of a cDNA for rat TM4SF4, a homolog of human il-TMP (TM4SF4), and enhanced expression of the corresponding gene in regenerating rat liver(1). Biochim. Biophys Acta 2001, 1518, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Liu, Z.; Da, L.; Li, Y.; Xuan, H.; Lin, Q.; Li, F.; Wang, Y.; Li, Z.; Zhao, M. Overexpression of the gene for transmembrane 4 superfamily member 4 accelerates liver damage in rats treated with CCl4. J. Hepatol. 2007, 46, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lee, S.; Cho, I.; Oh, M.; Kang, E.; Kim, Y.; Seo, W.; Choi, S.; Nam, J.; Tamamori, A.; et al. Tetraspanin TM4SF5 mediates loss of contact inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J. Clin. Invest. 2008, 118, 1354–1366. [Google Scholar] [CrossRef] [Green Version]
- Muller-Pillasch, F.; Wallrapp, C.; Lacher, U.; Friess, H.; Buchler, M.; Adler, G.; Gress, T. Identification of a new tumour-associated antigen TM4SF5 and its expression in human cancer. Gene 1998, 208, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Le Tallec, L.; Dulmet, E.; Bertagna, X.; de Keyzer, Y. Identification of genes associated with the corticotroph phenotype in bronchial carcinoid tumors. J. Clin. Endocrinol. Metab. 2002, 87, 5015–5022. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, J.W. Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5. Genom. Inf. 2014, 12, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, M.; Wang, B.; Liu, M.; Jiang, K.; Hou, X.; Wang, L. A preliminary attempt to explore the potential functions of a tetraspanin gene (MmTSPAN) in the innate immunity of hard clam Meretrix meretrix: Sequence features and expression profiles. Fish Shellfish. Immunol. 2019, 88, 135–141. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Detchokul, S.; Williams, E.; Parker, M.; Frauman, A. Tetraspanins as regulators of the tumour microenvironment: Implications for metastasis and therapeutic strategies. Br. J. Pharmacol. 2014, 171, 5462–5490. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Mo, M.; Barreiro, O.; Gordon-Alonso, M.; Sala-Valdes, M.; Sanchez-Madrid, F. Tetraspanin-enriched microdomains: A functional unit in cell plasma membranes. Trends Cell Biol. 2009, 19, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.-M.H.; Amoussou, N.; Mai, H.; Logé, C.; Brouard, S. Tetraspanins: Useful multifunction proteins for the possible design and development of small-molecule therapeutic tools. Drug Discov. Today 2020, 26, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Kim, S.-Y.; Lee, J.; Cho, E.-W.; Kim, I.-G. TM4SF4 overexpression in radiation-resistant lung carcinoma cells activates IGF1R via elevation of IGF1. Oncotarget 2014, 5, 9823–9837. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, Q.; Xu, D.; Wang, Q.; An, Y.; Du, Q.; Zhang, J.; Zhu, Y.; Miao, Y. hsa-miR-141 downregulates TM4SF1 to inhibit pancreatic cancer cell invasion and migration. Int. J. Oncol. 2014, 44, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xu, Y.; Xu, J.; Lu, D.; Wang, J. Role of TM4SF1 in regulating breast cancer cell migration and apoptosis through PI3K/AKT/mTOR pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 9081–9088. [Google Scholar]
- Park, Y.R.; Lee, S.; Kim, S.; Liu, Y.; Lee, M.; Shin, J.; Seo, S.; Kim, S.; Kim, I.; Lee, S.; et al. MicroRNA-9 suppresses cell migration and invasion through downregulation of TM4SF1 in colorectal cancer. Int. J. Oncol. 2016, 48, 2135–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Shen, X.; Li, L.; Cao, G.; Cai, X.; Wang, Y.; Shen, H. TM4SF1 inhibits apoptosis and promotes proliferation, migration and invasion in human gastric cancer cells. Oncol. Lett. 2018, 16, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Kim, S.; Lee, J.; Kim, J.; Cho, E.; Kim, I. Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget 2017, 8, 101284–101297. [Google Scholar] [CrossRef]
- Wang, L.; Feng, L.; Da, Y.; Li, Z.; Zhao, M. Adenovirus-mediated delivery of siRNA targeting TM4SF4 attenuated liver cancer cell growth in vitro and in vivo. Acta Biochim. Biophys. Sin. 2013, 45, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Choi, K.; Kim, Y.; Ha, Y.; Kim, D.; Park, B.; Wu, G.; Kim, D.; Lee, Y.; Kwon, H. Monoclonal antibody targeting of the cell surface molecule TM4SF5 inhibits the growth of hepatocellular carcinoma. Cancer Res. 2014, 74, 3844–3856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W. Transmembrane 4 L Six Family Member 5 (TM4SF5)-Mediated Epithelial-Mesenchymal Transition in Liver Diseases. Int. Rev. Cell Mol. Biol. 2015, 319, 141–163. [Google Scholar]
- Park, B.K.; Park, J.; Kim, T.; Kim, D.; Wu, G.; Gautam, A.; Maharjan, S.; Lee, S.; Lee, Y.; Kwon, H.; et al. Production of an anti-TM4SF5 monoclonal antibody and its application in the detection of TM4SF5 as a possible marker of a poor prognosis in colorectal cancer. Int. J. Oncol. 2018, 53, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.; Wu, G.; Jung, H.; Park, J.; Kwon, H.; Lee, Y. A peptide-CpG-DNA-liposome complex vaccine targeting TM4SF5 suppresses growth of pancreatic cancer in a mouse allograft model. Onco. Targets Ther. 2018, 11, 8655–8672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.I.; Merley, A.; Sciuto, T.; Li, D.; Dvorak, A.; Melero-Martin, J.; Dvorak, H.; Jaminet, S. A new vascular therapeutic target in cancer. Angiogenesis 2014, 17, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Yang, J.; Ramachandran, V.; Arumugam, T.; Deng, D.; Li, Z.; Xu, L.; Logsdon, C. TM4SF1 Promotes Gemcitabine Resistance of Pancreatic Cancer In Vitro and In Vivo. PLoS ONE 2015, 10, e0144969. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Z.; Zhu, H.; Yu, X. Autophagy Regulatory Genes MET and RIPK2 Play a Prognostic Role in Pancreatic Ductal Adenocarcinoma: A Bioinformatic Analysis Based on GEO and TCGA. BioMed Res. Int. 2020, 2020, 8537381. [Google Scholar] [CrossRef]
- Fu, F.; Yang, X.; Zheng, M.; Zhao, Q.; Zhang, K.; Li, Z.; Zhang, H.; Zhang, S. Role of Transmembrane 4 L Six Family 1 in the Development and Progression of Cancer. Autophagy 2020, 7, 202. [Google Scholar] [CrossRef]
- Xu, D.; Yang, F.; Wu, K.; Xu, X.; Zeng, K.; An, Y.; Xu, F.; Xun, J.; Lv, X.; Zhang, X.; et al. Lost miR-141 and upregulated TM4SF1 expressions associate with poor prognosis of pancreatic cancer: Regulation of EMT and angiogenesis by miR-141 and TM4SF1 via AKT. Cancer Biol Ther. 2020, 21, 354–363. [Google Scholar] [CrossRef]
- Marken, J.S.; Schieven, G.; Hellström, I.; Hellström, K.; Aruffo, A. Cloning and expression of the tumor-associated antigen L6. Proc. Natl. Acad. Sci. USA 1992, 89, 3503–3507. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.; Hemler, M. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef]
- Shih, S.C.; Zukauskas, A.; Li, D.; Liu, G.; Ang, L.; Nagy, J.; Brown, L.; Dvorak, H. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009, 69, 3272–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.C.; Zhang, Y.; He, S.; Li, M.; Cai, X.; Wang, H.; Xu, L.; Cao, J. TM4SF1 Promotes Metastasis of Pancreatic Cancer via Regulating the Expression of DDR1. Sci. Rep. 2017, 7, 45895. [Google Scholar] [CrossRef]
- Zukauskas, A.; Merley, A.; Li, D.; Ang, L.; Sciuto, T.; Salman, S.; Dvorak, A.; Dvorak, H.; Jaminet, S. TM4SF1: A tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration. Angiogenesis 2011, 14, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.K.; Fan, X.; Qiu, F. TM4SF1 Promotes Proliferation, Invasion, and Metastasis in Human Liver Cancer Cells. Int. J. Mol. Sci. 2016, 17, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audet-Walsh, É.; Yee, T.; Tam, I.; Giguère, V. Inverse Regulation of DHT Synthesis Enzymes 5α-Reductase Types 1 and 2 by the Androgen Receptor in Prostate Cancer. Endocrinology 2017, 158, 1015–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, T.D.; Ashby, W.; Lewis, J.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, F.; Xu, H.; Chen, D.; Liu, W.; Wang, J. Over-expression of TM4SF1 improves cell metastasis and growth by activating ERK1/2 signaling pathway in human prostate cancer. J. Buon 2019, 24, 2531–2538. [Google Scholar]
- Peng, X.C.; Zeng, Z.; Huang, Y.; Deng, Y.; Fu, G. Clinical significance of TM4SF1 as a tumor suppressor gene in gastric cancer. Cancer Med. 2018, 7, 2592–2600. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanda, M.; Kodera, Y. Incorporating molecular biomarkers into clinical practice for gastric cancer. Expert Rev. Anticancer. Ther. 2019, 19, 757–771. [Google Scholar] [CrossRef]
- Gao, H.; Chakraborty, G.; Zhang, Z.; Akalay, I.; Gadiya, M.; Gao, Y.; Sinha, S.; Hu, J.; Jiang, C.; Akram, M.; et al. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell 2016, 166, 47–62. [Google Scholar] [CrossRef]
- Xing, P.; Dong, H.; Liu, Q.; Zhao, T.; Yao, F.; Xu, Y.; Chen, B.; Zheng, X.; Wu, Y.; Jin, F.; et al. Upregulation of transmembrane 4 L6 family member 1 predicts poor prognosis in invasive breast cancer: A STROBE-compliant article. Medicine 2017, 96, e9476. [Google Scholar] [CrossRef]
- Cao, R.; Wang, G.; Qian, K.; Chen, L.; Ju, L.; Qian, G.; Wu, C.; Dan, H.; Jiang, W.; Wu, M.; et al. TM4SF1 regulates apoptosis, cell cycle and ROS metabolism via the PPARgamma-SIRT1 feedback loop in human bladder cancer cells. Cancer Lett. 2018, 414, 278–293. [Google Scholar] [CrossRef]
- Omisanjo, O.A.; Ogunremi, O.; Akinola, O.; Adebayo, O.; Ojewuyi, O.; Omorinde, M.; Abolarinwa, A.; Ikuerowo, S.; Balogun, F. Waiting Times for Prostate Cancer Diagnosis in a Nigerian Population. J. Cancer Epidemiol. 2021, 2021, 5534683. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Lin, P.-H.; Shao, I.; Chu, Y.-C.; Kan, H.-C.; Liu, C.-Y.; Yu, K.-J.; Chang, Y.-H.; Pang, S.-T.; Huang, J.-L.; et al. Prostate-Specific Antigen Kinetics Effects on Outcomes of Low-Volume Metastatic Prostate Cancer Patients Receiving Androgen Deprivation Therapy. J. Oncol. 2021, 2021, 9648579. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Ricke, W.; Thomson, A.; Marker, P.; Risbridger, G.; Hayward, S.; Wang, Y.; Donjacour, A.; Kurita, T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid. Biochem. Mol. Biol. 2004, 92, 221–236. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Ross, K.; Balk, S. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006, 66, 7783–7792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhler, P.; Fischer, T.; Wolf, P.; Gierschner, D.; Schultze-Seemann, W.; Wetterauer, U.; Elsasser-Beile, U. Comparison of gene expression in LNCaP prostate cancer cells after treatment with bicalutamide or 5-alpha-reductase inhibitors. Urol. Int. 2010, 84, 203–211. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, Q. The evolving Gleason grading system. Chin. J. Cancer Res. 2016, 28, 58–64. [Google Scholar]
- Huo, Y.; Yang, M.; Liu, W.; Yang, J.; Fu, X.; Liu, D.; Li, J.; Zhang, J.; Hua, R.; Sun, Y. High expression of DDR1 is associated with the poor prognosis in Chinese patients with pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 88. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, R.; Tsuji, N.; Kamagata, C.; Endoh, T.; Nakamura, M.; Kobayashi, D.; Yagihashi, A.; Watanabe, N. Amount of expression of the tumor-associated antigen L6 gene and transmembrane 4 superfamily member 5 gene in gastric cancers and gastric mucosa. Am. J. Gastroenterol. 2001, 96, 3457–3458. [Google Scholar] [CrossRef]
- Xue, S.; Zhao, Q.; Tai, M.; Li, N.; Liu, Y. Correlation between Breast Ultrasound Microcalcification and the Prognosis of Breast Cancer. J. Healthc. Eng. 2021, 2021, 6835963. [Google Scholar] [CrossRef]
- Yang, W.-J.; Zhang, G.-L.; Cao, K.-X.; Yang, G.-W. A Hypercoagulable Hematological Metastasis Breast Cancer Model. BioMed Res. Int. 2021, 2021, 5473959. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liu, N.; Zheng, D.; Du, J.; Wang, K. MicroRNA-206 inhibits metastasis of triple-negative breast cancer by targeting transmembrane 4 L6 family member 1. Cancer Manag. Res. 2019, 11, 6755–6764. [Google Scholar] [CrossRef] [Green Version]
- Welf, E.S.; Haugh, J.M. Signaling pathways that control cell migration: Models and analysis. Wiley Interdiscip. Reviews. Syst. Biol. Med. 2011, 3, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Yu, J.; Jiang, Z.; Zhao, N. Oleanolic Acid (OA) Targeting UNC5B Inhibits Proliferation and EMT of Ovarian Cancer Cell and Increases Chemotherapy Sensitivity of Niraparib. J. Oncol. 2022, 2022, 5887671. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, L.; Xue, Q.; Tang, C.; Tang, W.; Zhang, N.; Dai, C.; Chen, Z. Novel lncRNA AL033381,2 Promotes Hepatocellular Carcinoma Progression by Upregulating PRKRA Expression. Oxidative Med. Cell. Longev. 2022, 2022, 1125932. [Google Scholar]
- Zhu, C.; Luo, X.; Wu, J.; Liu, Y.; Liu, L.; Ma, S.; Xie, R.; Wang, S.; Ji, W. TM4SF1, a binding protein of DVL2 in hepatocellular carcinoma, positively regulates beta-catenin/TCF signalling. J. Cell Mol. Med. 2021, 25, 2356–2364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qiao, L.; Liu, Q.; Kong, X.; Hu, J.; Hu, W.; Wu, Z.; Li, M.; Liu, L. Hypoxia associated multi-omics molecular landscape of tumor tissue in patients with hepatocellular carcinoma. Aging 2021, 13, 6525–6553. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Sun, D.; Wang, L.; Fan, R.; Gao, Z. Deep sequencing and comprehensive expression analysis identifies several molecules potentially related to human poorly differentiated hepatocellular carcinoma. FEBS Open Bio. 2017, 7, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fan, W.; Chen, Y. microRNA-520f inhibits hepatocellular carcinoma cell proliferation and invasion by targeting TM4SF1. Gene 2018, 657, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ryu, J.; Lee, D.; Lee, M.; Kim, H.; Nam, S.; Song, H.; Choi, J.; Lee, G.; Kim, T.; et al. Correlations between transmembrane 4 L6 family member 5 (TM4SF5), CD151, and CD63 in liver fibrotic phenotypes and hepatic migration and invasive capacities. PLoS ONE 2014, 9, e102817. [Google Scholar] [CrossRef] [Green Version]
- Visintin, A.; Knowlton, K.; Tyminski, E.; Lin, C.; Zheng, X.; Marquette, K.; Jain, S.; Tchistiakova, L.; Li, D.; O’Donnell, C.J.; et al. Novel Anti-TM4SF1 Antibody-Drug Conjugates with Activity against Tumor Cells and Tumor Vasculature. Mol. Cancer Ther. 2015, 14, 1868–1876. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.; Wu, Y.; Sekar, M.; Chitranshi, N.; Malviya, R.; et al. Comprehensive Review of Methodology to Detect Reactive Oxygen Species (ROS) in Mammalian Species and Establish Its Relationship with Antioxidants and Cancer. Antioxidants 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.R.; Singer, D.A.; Balderes, L.; Hernandez-Lagunas, C.W.; Johnson, K.B.; Artinger, A.; Sussel, L. The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration. Development 2011, 138, 3213–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Choi, J.-S.; Koo, B.-M.; Kim, Y.; Song, J.-Y.; Sung, M.; Chang, E.; Noh, K.-W.; An, S.; Lee, M.-S.; et al. TM4SF4 and LRRK2 Are Potential Therapeutic Targets in Lung and Breast Cancers through Outlier Analysis. Cancer Res. Treat. 2021, 53, 9–24. [Google Scholar] [CrossRef]
- You, S.; Gao, L. Identification of NMU as a potential gene conferring alectinib resistance in non-small cell lung cancer based on bioinformatics analyses. Gene 2018, 678, 137–142. [Google Scholar] [CrossRef]
- Li, H.; Zhong, A.; Li, S.; Meng, X.; Wang, X.; Xu, F.; Lai, M. The integrated pathway of TGFbeta/Snail with TNFalpha/NFkappaB may facilitate the tumor-stroma interaction in the EMT process and colorectal cancer prognosis. Sci. Rep. 2017, 7, 4915. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, L.; Qiu, J.; Da, L.; Tiollais, P.; Li, Z.; Zhao, M. Human tetraspanin transmembrane 4 superfamily member 4 or intestinal and liver tetraspan membrane protein is overexpressed in hepatocellular carcinoma and accelerates tumor cell growth. Acta Biochim. Et Biophys. Sin. 2012, 44, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Gruijs, M.; Braster, R.; Overdijk, M.; Hellingman, T.; Verploegen, S.; Korthouwer, R.; van der Wilk, B.; Parren, P.; van der Vliet, H.; Bögels, M.; et al. Epidermal Growth Factor Receptor as Target for Perioperative Elimination of Circulating Colorectal Cancer Cells. J. Oncol. 2022, 2022, 3577928. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wu, F.; Fu, K.; Sun, G.; Sun, G.; Li, X.; Jiang, W.; Cao, H.; Wang, H.; Tang, W. Emerging Mechanisms and Treatment Progress on Liver Metastasis of Colorectal Cancer. OncoTargets Ther. 2021, 14, 3013–3036. [Google Scholar] [CrossRef]
- Son, H.; Moon, A. Epithelial-mesenchymal Transition and Cell Invasion. Toxicol. Res. 2010, 26, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Neagu, M.; Constantin, C.; Bostan, M.; Caruntu, C.; Ignat, S.; Dinescu, S.; Costache, M. Proteomic Technology “Lens” for Epithelial-Mesenchymal Transition Process Identification in Oncology. Anal. Cell. Pathol. 2019, 2019, 3565970. [Google Scholar] [CrossRef] [PubMed]
- Medrano-González, P.A.; Rivera-Ramírez, O.; Montaño, L.; Rendón-Huerta, E. Proteolytic Processing of CD44 and Its Implications in Cancer. Stem Cells Int. 2021, 2021, 6667735. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Ryu, H.; Kim, Y.; Choi, S.; Lee, M.; Kwak, T.; Kim, H.; Cho, M.; Park, K.; Lee, J. Blockade of four-transmembrane L6 family member 5 (TM4SF5)-mediated tumorigenicity in hepatocytes by a synthetic chalcone derivative. Hepatology 2009, 49, 1316–1325. [Google Scholar] [CrossRef]
- Wu, Y.B.; Huang, Y.; Xu, Y.; Sun, Y.; Yu, D.; Zhang, X.; Long, X.; Zhu, S.; Zhou, J.; Xu, J. A high level of TM4SF5 is associated with human esophageal cancer progression and poor patient survival. Dig. Dis. Sci. 2013, 58, 2623–2633. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.W. Self-renewal and circulating capacities of metastatic hepatocarcinoma cells required for collaboration between TM4SF5 and CD44. BMB Rep. 2015, 48, 127–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kang, M.; Lee, S.; Kwak, T.; Jung, O.; Lee, H.; Kim, S.; Lee, J. TM4SF5 accelerates G1/S phase progression via cytosolic p27Kip1 expression and RhoA activity. Biochim. Biophys Acta 2010, 1803, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Lee, M.; Ryu, H.; Kwak, T.; Kim, H.; Kang, M.; Jung, O.; Kim, H.; Park, K.; Lee, J. Differential inhibition of transmembrane 4 L six family member 5 (TM4SF5)-mediated tumorigenesis by TSAHC and sorafenib. Cancer Biol. Ther. 2011, 11, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Na, J.; Ryu, J.; Kim, H.; Nam, S.; Kang, M.; Jung, J.; Lee, M.; Song, H.; Choi, J.; et al. Interaction of tetraspan(in) TM4SF5 with CD44 promotes self-renewal and circulating capacities of hepatocarcinoma cells. Hepatology 2015, 61, 1978–1997. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.; Kwak, T.; Kim, H.; Lee, M.; Ye, S.; Kim, S.; Kim, S.; Lee, J. Cooperation between integrin alpha5 and tetraspan TM4SF5 regulates VEGF-mediated angiogenic activity. Blood 2009, 113, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, T.; Kwak, T.; Kim, H.; Kim, S.; Lee, H.; Kim, S.; Park, K.; Kim, H.; Cho, M.; et al. Transmembrane 4 L six family member 5 (TM4SF5) enhances migration and invasion of hepatocytes for effective metastasis. J. Cell Biochem. 2010, 111, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Choi, S.; Jeong, S.-J.; Lee, S.-A.; Kwak, T.K.; Kim, H.; Jung, O.; Lee, M.-S.; Ko, Y.; Ryu, J.; et al. Cross-talk between TGFβ1 and EGFR signalling pathways induces TM4SF5 expression and epithelial–mesenchymal transition. Biochem. J. 2012, 443, 691–700. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kwon, S.; Wu, G.; Kim, D.; Park, B.; Park, J.; Choi, K.; Kim, D.; Kwon, H.; Lee, Y. Therapeutic effect of a TM4SF5-specific monoclonal antibody against colon cancer in a mouse model. Oncotarget 2014, 5, 8402–8415. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Feng, J.; Zhu, Y.; Xie, X.; Huang, H.; Li, Y.; Lu, Q.; Jiang, J.; Wang, H. Clinicopathological and prognostic significance of TM4SF5 in colorectal cancer. J. Cancer 2021, 12, 1583–1591. [Google Scholar]
- Kim, Y.B.; Lee, S.; Ye, S.; Lee, J. Epigenetic regulation of integrin-linked kinase expression depending on adhesion of gastric carcinoma cells. Am. J. Physiol. Cell Physiol. 2007, 292, C857–C866. [Google Scholar] [CrossRef]
- Li, D.F.; Yang, M.; Shi, S.; Du, Y.; Wang, H.; Zhou, Y.; Luo, Y.; Ren, L.; Nie, Y. TM4SF5-CTD-2354A18.1-miR-4697-3P may play a key role in the pathogenesis of gastric cancer. Bratisl Lek Listy 2015, 116, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, C.; Li, J.; Szeles, A.; Gizatullin, R.; Kashuba, V.; Lushnikova, T.; Protopopov, A.; Kelve, M.; Kiss, H.; Kholodnyuk, I.; et al. Assignment of the ARHA and GPX1 genes to human chromosome bands 3p21. 3 by in situ hybridization and with somatic cell hybrids. Cytogenet. Cell Genet. 1997, 79, 228–230. [Google Scholar]
- Narumiya, S.; Thumkeo, D. Rho signaling research: History, current status and future directions. FEBS Lett. 2018, 592, 1763–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Seen, D.; Zheng, C.; Zeng, R.; Li, E. Role of Small GTPase RhoA in DNA Damage Response. Biomolecules 2021, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xie, F.; Miao, F.; Long, J.; Huang, S.; Huang, H.; Lin, J.; Wang, D.; Yang, X.; Bian, J.; et al. The diagnostic and prognostic role of RhoA in hepatocellular carcinoma. Aging 2019, 11, 5158–5172. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Choi, S.; Jang, S.; Lee, S.; Lim, S.; Choi, Y.; Kim, H.; Kim, D.; Kwak, T.; Kim, H.; et al. Tetraspan TM4SF5-dependent direct activation of FAK and metastatic potential of hepatocarcinoma cells. J. Cell Sci. 2012, 125(Pt 24) Pt 24, 5960–5973. [Google Scholar] [CrossRef] [Green Version]
- Sadeghian, Z.; Baghi, H.B.; Poortahmasebi, V.; Sadeghi, J.; Hasani, A.; Azadi, A.; Oskouee, M.A. Evidence of High-Risk Human Papillomavirus in Esophageal Cancer in East Azerbaijan Province, Northwest of Iran. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1099477. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.D.; Matassa, S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of Mitochondrial Metabolic Reprogramming and Oxidative Stress to Overcome Chemoresistance in Cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Kono, Y.; Itoh, S.; Inoue, T.; Urata, Y.; Kawa, Y.; Tohnai, R.; Kumagai, T.; Nishino, K.; Uozumi, R.; et al. A phase I/II study of weekly nab-paclitaxel plus cisplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer. BMC Cancer 2020, 20, 115. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Fan, Y.; Li, L.; He, X.; Xiang, Q.; Mu, J.; Zhou, D.; Sun, X.; Yang, Y.; et al. The new 6q27 tumor suppressor DACT2, frequently silenced by CpG methylation, sensitizes nasopharyngeal cancer cells to paclitaxel and 5-FU toxicity via beta-catenin/Cdc25c signaling and G2/M arrest. Clin. Epigenetics 2018, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Ongusaha, P.P.; Kim, J.; Fang, L.; Wong, T.; Yancopoulos, G.; Aaronson, S.; Lee, S. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loo. EMBO J. 2003, 22, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.J.; Zhou, Y.; Ning, J.; Yang, T.; Ren, H.; Li, Y.; Zhang, S.; Chen, M. NBM-T-BMX-OS01, an Osthole Derivative, Sensitizes Human Lung Cancer A549 Cells to Cisplatin through AMPK-Dependent Inhibition of ERK and Akt Pathway. Cell Physiol. Biochem. 2015, 36, 893–906. [Google Scholar] [CrossRef] [PubMed]
- El Azreq, M.A.; Kadiri, M.; Boisvert, M.; Page, N.; Tessier, P.; Aoudjit, F. Discoidin domain receptor 1 promotes Th17 cell migration by activating the RhoA/ROCK/MAPK/ERK signaling pathway. Oncotarget 2016, 7, 44975–44990. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, P.A.; Jarai, G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J. Biol. Chem. 2011, 286, 12912–12923. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Zhu, L.; Zhuang, H.; Hao, Y.; Gao, S.; Liu, S.; Liu, Q.; Liu, D.; Liu, J.; Lin, B. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am. J. Cancer Res. 2015, 5, 2777–2787. [Google Scholar] [PubMed]
- Lee, M.S.; Kim, H.; Kim, T.; Lee, J. Gefitinib resistance of cancer cells correlated with TM4SF5-mediated epithelial-mesenchymal transition. Biochim. Biophys. Acta 2012, 1823, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.M.; Allison, J.; Wolchok, J. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14. [Google Scholar] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jeong, S.; Park, S.; Lee, H.; Kim, H.; Park, K.; Ye, S.; Kim, S.; Lee, J. Antagonistic regulation of transmembrane 4 L6 family member 5 attenuates fibrotic phenotypes in CCl(4) -treated mice. FEBS J. 2012, 279, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.; Park, J.; Kwon, H.; Lee, Y. Targeting TM4SF5 with anti-TM4SF5 monoclonal antibody suppresses the growth and motility of human pancreatic cancer cells. Oncol Lett. 2020, 19, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Kim, Y.; Kim, D.; Park, B.; Wu, G.; Kim, T.; Choi, S.; Kim, D.; Kwon, H.; Lee, Y. Prophylactic effect of a peptide vaccine targeting TM4SF5 against colon cancer in a mouse model. Biochem. Biophys. Res. Commun. 2013, 435, 134–139. [Google Scholar] [CrossRef]
- Faria, C.; Fortunato, R. The role of dual oxidases in physiology and cancer. Genet. Mol. Biol. 2020, 43 (Suppl. 1), e20190096. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wu, Y.; Tunki, L.; Chellian, R.; Halmuthur, M.; Muller, S.; Pandy, V. What Has Come out from Phytomedicines and Herbal Edibles for the Treatment of Cancer? ChemMedChem 2018, 13, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Loh, W.; Gopinath, S.; Bonam, S.; Fareez, I.; Guad, R.M.; Sim, M.; Wu, Y. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm. Sin. B 2019. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, K.D.; Gilbert, J.; Jilma, B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv. Drug Deliv. Rev 2018, 134, 36–50. [Google Scholar] [CrossRef] [PubMed]





| Cancer Types | Test Model (In Vitro/In Vivo/Clinical) | Metabolic Responses/Mechanisms | Reference |
|---|---|---|---|
| Prostate | In vitro (PC-3, DU145, LNCaP and VCaP) |
| [27,70,71,72] |
| In vivo (Nude mice) |
| [59] | |
| Clinical (Human prostate tumor tissue) |
| [27] | |
| Pancreas | In vitro (AsPC-1, MIA PaCa-2, PANC-1, SW1990 and BxPc-3 cells) |
| [31,49,67] |
| Clinical (Human PC tissue) |
| [30,67] | |
| Gastric | In vitro (MGC803 and MKN45 cells) |
| [52] |
| Clinical (Gastric mucosa tissues) |
| [73,74] | |
| Breast | In vitro (MDA-MB-231 cells) |
| [50] |
| In vivo (Syngeneic BALB/c mice) |
| [75] | |
| Clinical (BC tumor) | 1. Low estrogen receptor (ER), low progesterone receptor (PR), and low human epidermal growth factor receptor 2 (HER2) expression were linked to high TM4SF1 expression in triple-negative breast cancer (TNBC).2. Disease-free survival (DFS) and OS were expected to be shorter in these patients. | [76] | |
| Ovarian | In vitro (HO8910PM and SKOV3 cells) |
| [13] |
| In vivo (Nude mice) |
| [13] | |
| Clinical (Epithelial OC tissues) |
| [13] | |
| Hepatocellular | In vitro (HepG2 and HUVEC cells) |
| [66,69] |
| In vivo (Foxn1−/− nude mice) |
| [69] | |
| Bladder | In vitro (T24, EJ and UM-UC-3 cells) |
| [77] |
| In vivo (NOD/SCID xenotransplanted tumor mice) |
| [77] | |
| Clinical (Human muscle invasive bladder cancer (MIBC) tissues) |
| [77] |
| Cancer Types | Test Model (In Vitro/In Vivo/Clinical) | Metabolic Responses/Mechanisms | Reference |
|---|---|---|---|
| Lung | In vitro (HCC-1833, A549 and Calu-3 cells) |
| [48,53,100] |
| In vivo (Athymic BALB/c nude mice) |
| [48] | |
| Clinical (LC tissue) |
| [100,101] | |
| Colorectal | Clinical (CRC tissue and CRC tumor buds) |
| [32,102] |
| Hepatocellular | In vitro (QGY-7701, SMMC-7721 and BEL-7404 cell) |
| [54,103] |
| In vivo (Xenograft tumor model nude mice) |
| [54] | |
| Clinical (HCC tissue) |
| [54,103] |
| Cancer Types | Test Model (In Vitro/In Vivo/Clinical) | Metabolic Responses/Mechanisms | Reference |
|---|---|---|---|
| Hepatocellular | In vitro (SNU449 and Huh7 cells) |
| [38,112,113,114,115] |
| In vivo (BALB/c-n/n mice) |
| [116] | |
| Clinical (Tumor liver tissues) |
| [38,112,115,117] | |
| Esophageal | In vitro (KYSE150 cells) |
| [110] |
| Pancreas | In vitro (PANC02 cells) |
| [58] |
| In vivo (C57BL/6 allograft mice model) |
| [58] | |
| Clinical (PC tissue) |
| [39] | |
| Colorectal | In vitro (CT-26, LoVo, and SW480 cells) |
| [118,119] |
| Clinical (CRC tissues) |
| [23,57] | |
| Gastric | In vitro (SNU601 cells) |
| [38,120] |
| Clinical (GC tissues) |
| [39,121] |
| TM4SF | Cancer Types | Test Model (In Vitro/In Vivo) | Metabolic Responses/Mechanisms | Reference |
|---|---|---|---|---|
| TM4SF1 | Lung | In vitro (A549 and H1299 cells) |
| [16] |
| Breast | In vitro (MDA-MB-231 cells) |
| [50] | |
| Pancreatic | In vitro (AsPC-1, MIA PaCa-2 and PANC-1 cell) |
| [60] | |
| In vivo (Athymic nude nu/nu mice) |
| [60] | ||
| TM4SF4 | Ovarian | In vitro (RMG-I-H, RMG-I, COC1/DDP and COC1 cells) |
| [17] |
| TM4SF5 | Lung | In vitro (Gefitinib-sensitive cells; HCC827, Gefitinib-resistant cells; NCI-H358) |
| [136] |
| Liver | In vitro (SNU449) |
| [115] | |
| In vivonihao(TM4SF5-overexpressing transgenic mice, zebrafish) |
| [41,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, N.S.; Wu, Y.S.; Sim, M.S.; Velaga, A.; Bonam, S.R.; Gopinath, S.C.B.; Subramaniyan, V.; Choy, K.W.; Teow, S.-Y.; Fareez, I.M.; et al. Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance. Pharmaceuticals 2023, 16, 110. https://doi.org/10.3390/ph16010110
Rahim NS, Wu YS, Sim MS, Velaga A, Bonam SR, Gopinath SCB, Subramaniyan V, Choy KW, Teow S-Y, Fareez IM, et al. Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance. Pharmaceuticals. 2023; 16(1):110. https://doi.org/10.3390/ph16010110
Chicago/Turabian StyleRahim, Nur Syafiqah, Yuan Seng Wu, Maw Shin Sim, Appalaraju Velaga, Srinivasa Reddy Bonam, Subash C. B. Gopinath, Vetriselvan Subramaniyan, Ker Woon Choy, Sin-Yeang Teow, Ismail M. Fareez, and et al. 2023. "Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance" Pharmaceuticals 16, no. 1: 110. https://doi.org/10.3390/ph16010110
APA StyleRahim, N. S., Wu, Y. S., Sim, M. S., Velaga, A., Bonam, S. R., Gopinath, S. C. B., Subramaniyan, V., Choy, K. W., Teow, S. -Y., Fareez, I. M., Samudi, C., Sekaran, S. D., Sekar, M., & Guad, R. M. (2023). Three Members of Transmembrane-4-Superfamily, TM4SF1, TM4SF4, and TM4SF5, as Emerging Anticancer Molecular Targets against Cancer Phenotypes and Chemoresistance. Pharmaceuticals, 16(1), 110. https://doi.org/10.3390/ph16010110