Methyleugenol Has an Antidepressant Effect in a Neuroendocrine Model: In Silico and In Vivo Evidence
Abstract
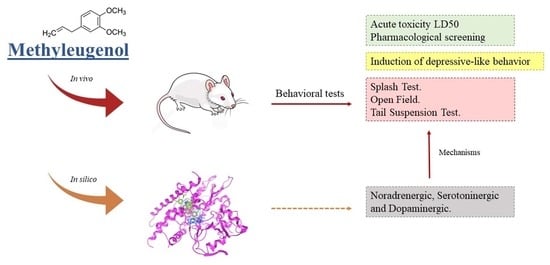
:1. Introduction
2. Results
2.1. Behavioral Pharmacology Screening and LD50 Estimation
2.2. Tail Suspension Test
2.3. Splash Test
2.4. Open Field Test
2.5. Assessment of the Mechanisms of Action in the Antidepressant Effects of Methyleugenol
2.6. In Silico Methodologies
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. Animals
4.3. In Vivo Methodologies
4.3.1. Methyleugenol Acute Toxicity Estimation and Pharmacological Screening
4.3.2. Induction of Depressive-like Behavior
Tail Suspension Test
Splash Test
Open Field Test
Investigation of the Mechanisms of Action of the Antidepressant Activity of Methyleugenol
4.4. Video Recording and Analysis
4.5. Statistical Analysis
4.6. In Silico Methodology
Investigation of the Mechanisms of Action of the Antidepressant Activity of Methyleugenol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pitsillou, E.; Bresnehan, S.M.; Kagarakis, E.A.; Wijoyo, S.J.; Liang, J.; Hung, A.; Karagiannis, T.C. The cellular and molecular basis of major depressive disorder: Towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020, 47, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Bakky, M.S.; Amin, E.; Faris, T.M.; Abdellatif, A.A.H. Mental depression: Relation to different disease status, newer treatments and its association with COVID-19 pandemic (Review). Mol. Med. Rep. 2021, 24, 839. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Salvioli, S.; Franceschi, C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013, 9, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Borah, L.; Lahkar, M.; Dasgupta, S. Study of the antidepressant activity of folic acid and vitamin-D on reserpine induced depression in mice. Asian J. Pharm. Clin. Res. 2018, 11, 255–259. [Google Scholar] [CrossRef]
- Dong, H.; Gao, Z.; Rong, H.; Jin, M.; Zhang, X. β-Asarone Reverses Chronic Unpredictable Mild Stress-Induced Depression-Like Behavior and Promotes Hippocampal Neurogenesis in Rats. Molecules 2014, 19, 5634–5649. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Huang, Z.; Zhong, X.M.; Xian, Y.F.; Ip, S.P. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 2014, 261, 140–145. [Google Scholar] [CrossRef]
- Sterner, E.Y.; Kalynchuk, L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: Relevance to depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 777–790. [Google Scholar] [CrossRef]
- Bao, A.-M.; Swaab, D.F. Corticotropin-Releasing Hormone and Arginine Vasopressin in Depression: Focus on the Human Postmortem Hypothalamus. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2010; pp. 339–365. [Google Scholar]
- Holzmann, I.; Da Silva, L.M.; Corrêa Da Silva, J.A.; Steimbach, V.M.B.; De Souza, M.M. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 2015, 136, 55–63. [Google Scholar] [CrossRef]
- Bai, Y.; Song, L.; Dai, G.; Xu, M.; Zhu, L.; Zhang, W.; Jing, W.; Ju, W. Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids 2018, 135, 73–78. [Google Scholar] [CrossRef]
- Fasipe, O.J. The emergence of new antidepressants for clinical use: Agomelatine paradox versus other novel agents. IBRO Rep. 2019, 6, 95–110. [Google Scholar] [CrossRef]
- Khushboo, S.B.; Sharma, B. Antidepressants: Mechanism of Action, Toxicity and Possible Amelioration. J. Appl. Biotechnol. Bioeng. 2017, 3, 437–448. [Google Scholar] [CrossRef]
- Khawam, E.A.; Laurencic, G.; Malone, D.A. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Lahlou, M. Screening of natural products for drug discovery. Expert Opin. Drug Discov. 2007, 2, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Ge, Y.; Liu, M. Ginkgo biloba extract reduces hippocampus inflammatory responses, improves cardiac functions and depressive behaviors in a heart failure mouse model. Neuropsychiatr. Dis. Treat. 2019, 15, 3041–3050. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Alpinia zerumbet, a ginger plant with a multitude of medicinal properties: An update on its research findings. J. Chin. Pharm. Sci. 2017, 26, 775–788. [Google Scholar] [CrossRef]
- Galdino, P.M.; Carvalho, A.A.V.; Florentino, I.F.; Martins, J.L.R.; Gazola, A.C.; De Paula, J.R.; De Paula, J.A.M.; Torres, L.M.B.; Costa, E.A.; De Lima, T.C.M. Involvement of monoaminergic systems in the antidepressant-like properties of Lafoensia pacari A. St. Hil. J. Ethnopharmacol. 2015, 170, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.-A.; Abbasi-Maleki, S.; Mousavi, Z. Anti-depressive-like effect of monoterpene trans-anethole via monoaminergic pathways. Saudi J. Biol. Sci. 2022, 29, 3255–3261. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Chalchat, J.-C. Essential oil composition of Ocimum basilicum L. and Ocimum minimum L. in Turkey. Czech J. Food Sci. 2002, 20, 223–228. [Google Scholar] [CrossRef]
- Lima, R.K.; Cardoso, M.D.G.; Moraes, J.C.; Carvalho, S.M.; Melo, B.A.; Vieira, S.S. Composição química e toxicidade de óleos essenciais para o pulgão-verde Schizaphis graminum (Rondani, 1852). Arq. Inst. Biol. 2014, 81, 22–29. [Google Scholar] [CrossRef]
- Irie, Y.; Itokazu, N.; Anjiki, N.; Ishige, A.; Watanabe, K.; Keung, W.M. Eugenol exhibits antidepressant-like activity in mice and induces expression of metallothionein-III in the hippocampus. Brain Res. 2004, 1011, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.; Criddle, D.N.; Coelho-De-Souza, A.N.; Monte, F.J.Q.; Jaffar, M.; Leal-Cardoso, J.H. Relaxant and antispasmodic actions of methyleugenol on guinea-pig isolated ileum. Planta Med. 2000, 66, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Chen, F.; Ling, X.; Huang, Y.; Zheng, X.; Tang, Q.; Tan, X. Inhibitory effect of methyleugenol on IgE-mediated allergic inflammation in RBL-2H3 cells. Mediat. Inflamm. 2015, 2015, 463530. [Google Scholar] [CrossRef] [PubMed]
- Norte, M.C.B.; Cosentino, R.M.; Lazarini, C.A. Effects of methyl-eugenol administration on behavioral models related to depression and anxiety, in rats. Phytomedicine 2005, 12, 294–298. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bayrami, Z.; Farzaei, F.; Aneva, I.; Das, S.K.; Patra, J.K.; Das, G.; Abdollahi, M. Poisoning by Medical Plants. Arch. Iran. Med. 2020, 23, 117–127. [Google Scholar]
- Lukas, G.; Brindle, S.D.; Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 1971, 178, 562–564. [Google Scholar]
- Nguyen, E.T.; Caldwell, J.L.; Streicher, J.; Ghisays, V.; Balmer, N.J.; Estrada, C.M.; Solomon, M.B. Differential effects of imipramine and CORT 118335 (Glucocorticoid receptor modulator/mineralocorticoid receptor antagonist) on brain-endocrine stress responses and depression-like behavior in female rats. Behav. Brain Res. 2018, 336, 99–110. [Google Scholar] [CrossRef]
- Canet, G.; Chevallier, N.; Zussy, C.; Desrumaux, C.; Givalois, L. Central Role of Glucocorticoid Receptors in Alzheimer’s Disease and Depression. Front. Neurosci. 2018, 12, 739. [Google Scholar] [CrossRef]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Piotr Wlaź, E.P. The depressogenic-like effect of acute and chronic treatment with 2 dexamethasone and its influence on the activity of antidepressant drugs 3 in the forced swim test in adult mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 243–248. [Google Scholar] [CrossRef]
- Conti, M.; Spulber, S.; Raciti, M.; Ceccatelli, S. Depressive-like phenotype induced by prenatal dexamethasone in mice is reversed by desipramine. Neuropharmacology 2017, 126, 242–249. [Google Scholar] [CrossRef]
- Carneiro, C.A.; Santos, A.M.F.; Guedes, É.C.; Cavalcante, I.L.; Santos, S.G.; Oliveira, A.M.F.; Barbosa, F.F.; Silva, M.S.; Almeida, R.N.; Salvadori, M.S. Unpredictable Subchronic Stress Induces Depressive-Like Behavior: Behavioral and Neurochemical Evidences. Psychol. Neurosci. 2022, 15, 236–250. [Google Scholar] [CrossRef]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011, 49, 5–8. [Google Scholar] [CrossRef]
- Can, Ö.D.; Turan, N.; Özkay, Ü.D.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Mitchell, P.J. The validity of animal models of predisposition to depression. Behav. Pharmacol. 2002, 13, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P. Rodent Models of Depression: Reexamining Validity Without Anthropomorphic Inference. Crit. Rev. Neurobiol. 2003, 15, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Castagné, V.; Porsolt, R.D.; Moser, P. Use of latency to immobility improves detection of antidepressant-like activity in the behavioral despair test in the mouse. Eur. J. Pharmacol. 2009, 616, 128–133. [Google Scholar] [CrossRef]
- Sigwalt, A.R.; Budde, H.; Helmich, I.; Glaser, V.; Ghisoni, K.; Lanza, S.; Cadore, E.L.; Lhullier, F.L.R.; de Bem, A.F.; Hohl, A.; et al. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience 2011, 192, 661–674. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Wlaź, P.; Poleszak, E. The effect of imipramine, ketamine, and zinc in the mouse model of depression. Metab. Brain Dis. 2015, 30, 1379–1386. [Google Scholar] [CrossRef]
- Moretti, M.; Colla, A.; De Oliveira Balen, G.; Dos Santos, D.B.; Budni, J.; De Freitas, A.E.; Farina, M.; Severo Rodrigues, A.L. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J. Psychiatr. Res. 2012, 46, 331–340. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Camargo, A.; Dalmagro, A.P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 2017, 125, 131–136. [Google Scholar] [CrossRef] [PubMed]
- de Souza, I.B.M.B.; Costa, L.R.F.; Tiago, P.R.F.; Cagni, F.C.; Lima, R.H.; Silva Junior, E.D.; Gavioli, E.C. Venlafaxine and Nortriptyline Reverse Acute Dexamethasone-Induced Depressive-Like Behaviors in Male and Female Mice. Exp. Clin. Psychopharmacol. 2019, 27, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.L.S.; Rocha, J.B.T.; Mello, C.F.; Souza, D.O. Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacol. Toxicol. 1996, 79, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Ge, H.; Bian, Y.; He, X.; Xie, X.Q.; Wang, J. Significantly different effects of tetrahydroberberrubine enantiomers on dopamine D1/D2 receptors revealed by experimental study and integrated in silico simulation. J. Comput. Aided. Mol. Des. 2019, 33, 447–459. [Google Scholar] [CrossRef]
- Qian, W.; Lu, W.; Sun, H.; Li, Z.; Zhu, L.; Zhao, R.; Zhang, L.; Zhou, S.; Zhou, Y.; Jiang, H.; et al. Design, synthesis, and pharmacological evaluation of novel tetrahydroprotoberberine derivatives: Selective inhibitors of dopamine D1 receptor. Bioorg. Med. Chem. 2012, 20, 4862–4871. [Google Scholar] [CrossRef]
- Specker, E.; Matthes, S.; Wesolowski, R.; Schütz, A.; Grohmann, M.; Alenina, N.; Pleimes, D.; Mallow, K.; Neuenschwander, M.; Gogolin, A.; et al. Structure-Based Design of Xanthine-Benzimidazole Derivatives as Novel and Potent Tryptophan Hydroxylase Inhibitors. J. Med. Chem. 2022, 65, 11126–11149. [Google Scholar] [CrossRef]
- Brunello, N.; Blier, P.; Judd, L.L.; Mendlewicz, J.; Nelson, C.J.; Souery, D.; Zohar, J.; Racagni, G. Noradrenaline in mood and anxiety disorders: Basic and clinical studies. Int. Clin. Psychopharmacol. 2003, 18, 191–202. [Google Scholar] [CrossRef]
- Kitada, Y.; Miyauchi, T.; Kanazawa, Y.; Nakamichi, H.; Satoh, S. Involvement of α- and β1-adrenergic mechanisms in the immobility-reducing action of desipramine in the forced swimming test. Neuropharmacology 1983, 22, 1055–1060. [Google Scholar] [CrossRef]
- Masuda, Y.; Ohnuma, S.; Sugiyama, T. α2-Adrenoceptor activity induces the antidepressant-like glycolipid in mouse forced swimming. Methods Find. Exp. Clin. Pharmacol. 2001, 23, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Chellian, R.; Pandy, V.; Mohamed, Z. Biphasic effects of α-asarone on immobility in the tail suspension test: Evidence for the involvement of the noradrenergic and serotonergic systems in its antidepressant-like activity. Front. Pharmacol. 2016, 7, 72. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Abbasi-Maleki, S.; Mousavi, Z. Hydroethanolic extract of Carthamus tinctorius induces antidepressant-like effects: Modulation by dopaminergic and serotonergic systems in tail suspension test in mice. Iran. J. Basic Med. Sci. 2017, 20, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. Evolving concepts of emotion and motivation. Front. Psychol. 2018, 9, 1647. [Google Scholar] [CrossRef]
- Millan, M.J.; Lejeune, F.; Gobert, A.; Brocco, M.; Auclair, A.; Bosc, C.; Rivet, J.M.; Lacoste, J.M.; Cordi, A.; Dekeyne, A. S18616, a highly potent spiroimidazoline agonist at alpha(2)-adrenoceptors: II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. J. Pharmacol. Exp. Ther. 2000, 295, 1206–1222. [Google Scholar] [PubMed]
- Naranjo, C.A.; Tremblay, L.K.; Busto, U.E. The role of the brain reward system in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 781–823. [Google Scholar] [CrossRef]
- Lehr, E. Potential antidepressant properties of pramipexole detected in locomotor and operant behavioral investigations in mice. Psychopharmacology 2002, 163, 495–500. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Marasca, C.; Cavalli, A.; Serretti, A.; Mercolini, L. New-generation, non-SSRI antidepressants: Drug-drug interactions and therapeutic drug monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and others. Med. Res. Rev. 2020, 40, 1794–1832. [Google Scholar] [CrossRef]
- ANVISA. ANVISA—Agência Nacional de Vigilância Sanitária. Available online: https://portal.anvisa.gov.br/wps/portal/anvisa/home (accessed on 20 October 2022).
- OECD. The Organization of Economic Co-Operation and Development Guidelines Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Thierry, B.; Simon, P.; Porsolt, R.D. Psychopharmacology The tail suspension test: Ethical considerations. Psychopharmacology 1986, 90, 284–285. [Google Scholar] [CrossRef]
- Broadhurst, P.L. Experiments in psychogenetics. In Experiments in Personality; Routledge & Kegan Paul Archive: London, UK, 1960; pp. 31–71. [Google Scholar]
- Teng, X.; Chen, S.; Nie, Y.; Xiao, P.; Yu, X.; Shao, Z.; Zheng, S. Ligand recognition and biased agonism of the D1 dopamine receptor. Nat. Commun. 2022, 13, 3186. [Google Scholar] [CrossRef] [PubMed]
- Deluigi, M.; Morstein, L.; Schuster, M.; Klenk, C.; Merklinger, L.; Cridge, R.R.; de Zhang, L.A.; Klipp, A.; Vacca, S.; Vaid, T.M.; et al. Crystal structure of the α1B-adrenergic receptor reveals molecular determinants of selective ligand recognition. Nat. Commun. 2022, 13, 382. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Satou, K.; Hirota, H. Evaluations of molecular docking programs for virtual screening. J. Chem. Inf. Model. 2007, 47, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Dassault Systèmes. BIOVIA Discovery Studio Client, Version BIOVIA 2021. San Diego: Dassault Systèmes. 2023. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 1 January 2023).







| Molecules | D1 Dopaminergic | α1 Adrenergic | Tryptophan Hydroxylase | |||
|---|---|---|---|---|---|---|
| Kcal/mol | Stand. 1 | Kcal/mol | Stand. | Kcal/mol | Stand. | |
| Methyleugenol | −25.1705 | −0.1412 | −63.7474 | −0.3576 | −76.295 | −0.428 |
| Prazosin 2 | - | - | −120.102 | −0.3132 | - | - |
| (+)-Cyclazosin 3 | - | - | −135.19 | −0.309 | - | - |
| SCH 23390 4 | −31.5101 | −0.11 | - | - | - | - |
| Fenoldopam 5 | −85.5469 | −0.2798 | - | - | - | - |
| PCPA 6 | - | - | - | - | −82.953 | −0.4155 |
| LP533401 7 | - | - | - | - | −130.797 | −0.2484 |
| RMSD | 0.19 | 0.3 | 0.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.C.N.; Cavalcante, I.L.; de Araújo, A.N.; Ferreira dos Santos, A.M.; de Menezes, R.P.B.; Herrera-Acevedo, C.; Ferreira de Sousa, N.; de Souza Aquino, J.; Barbosa-Filho, J.M.; de Castro, R.D.; et al. Methyleugenol Has an Antidepressant Effect in a Neuroendocrine Model: In Silico and In Vivo Evidence. Pharmaceuticals 2023, 16, 1408. https://doi.org/10.3390/ph16101408
Oliveira MCN, Cavalcante IL, de Araújo AN, Ferreira dos Santos AM, de Menezes RPB, Herrera-Acevedo C, Ferreira de Sousa N, de Souza Aquino J, Barbosa-Filho JM, de Castro RD, et al. Methyleugenol Has an Antidepressant Effect in a Neuroendocrine Model: In Silico and In Vivo Evidence. Pharmaceuticals. 2023; 16(10):1408. https://doi.org/10.3390/ph16101408
Chicago/Turabian StyleOliveira, Mayara Cecile Nascimento, Ikla Lima Cavalcante, Alana Natalícia de Araújo, Aline Matilde Ferreira dos Santos, Renata Priscila Barros de Menezes, Chonny Herrera-Acevedo, Natália Ferreira de Sousa, Jailane de Souza Aquino, José Maria Barbosa-Filho, Ricardo Dias de Castro, and et al. 2023. "Methyleugenol Has an Antidepressant Effect in a Neuroendocrine Model: In Silico and In Vivo Evidence" Pharmaceuticals 16, no. 10: 1408. https://doi.org/10.3390/ph16101408
APA StyleOliveira, M. C. N., Cavalcante, I. L., de Araújo, A. N., Ferreira dos Santos, A. M., de Menezes, R. P. B., Herrera-Acevedo, C., Ferreira de Sousa, N., de Souza Aquino, J., Barbosa-Filho, J. M., de Castro, R. D., Almeida, R. N., Scotti, L., Scotti, M. T., & Da Silva Stiebbe Salvadori, M. G. (2023). Methyleugenol Has an Antidepressant Effect in a Neuroendocrine Model: In Silico and In Vivo Evidence. Pharmaceuticals, 16(10), 1408. https://doi.org/10.3390/ph16101408