The Synthesis, In Vitro Bio-Evaluation, and In Silico Molecular Docking Studies of Pyrazoline–Thiazole Hybrid Analogues as Promising Anti-α-Glucosidase and Anti-Urease Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activities
2.2.1. In Vitro α-Glucosidase and Urease Inhibitory Potential
2.2.2. Structure–Activity Relationship (SAR) for α-Glucosidase and Urease Inhibitory
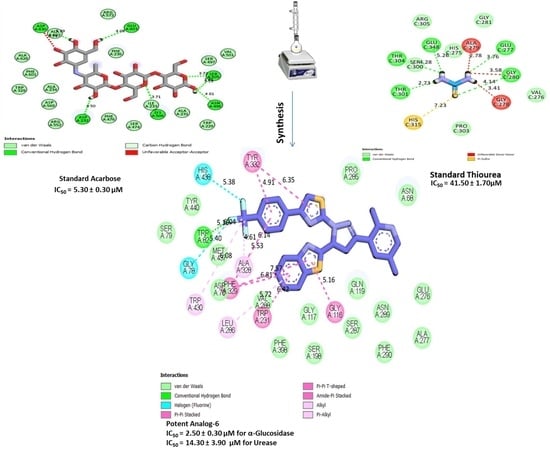
2.3. Molecular Docking Studies
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Chalcone
3.2.1. Synthesis of Chalcone Derivatives
3.2.2. Synthesis of Pyrazoline
3.2.3. Synthesis of Pyrazoline Derivatives with Phenacyl Bromide
3.3. Spectral Analysis
3.3.1. 2-(1-(4-(4-Bromo-2-nitrophenyl)thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (1)
3.3.2. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(2,5-dimethylphenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (2)
3.3.3. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(p-tolyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (3)
3.3.4. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(4-nitrophenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (4)
3.3.5. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(2-nitrophenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (5)
3.3.6. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(4-(trifluoromethyl)phenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (6)
3.3.7. 2-(2-(5-(Benzo[d]thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-5-fluorophenol (7)
3.3.8. 4-(2-(5-(Benzo[d]thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-N,N-dimethylaniline (8)
3.3.9. 2-(1-(4-(4-Chloro-2-nitrophenyl)thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (9)
3.3.10. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(2,5-dimethoxyphenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (10)
3.3.11. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(2-fluorophenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (11)
3.3.12. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(4-fluorophenyl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (12)
3.3.13. 2-(1-(4-(4-(Benzyloxy)phenyl)thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (13)
3.3.14. 2-(2-(5-(Benzo[d]thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazol-4-yl)-3,5-dichlorophenol (14)
3.3.15. 2-(1-(4-(4-Bromo-3,5-dimethylphenyl)thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (15)
3.3.16. 2-(3-(2,5-Dichloropyridin-3-yl)-1-(4-(naphthalen-2-yl)thiazol-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (16)
3.3.17. 2-(1-(4-(2,3-Dichlorophenyl)thiazol-2-yl)-3-(2,5-dichloropyridin-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzo[d]thiazole (17)
3.4. Molecular Modelling Assay
3.5. α-Glucosidase Inhibitory Assay
3.6. Urease Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshad, T.; Khan, K.M.; Rasool, N.; Salar, U.; Hussain, S.; Asghar, H.; Ashraf, M.; Wadood, A.; Riaz, M.; Perveen, S.; et al. 5-Bromo-2-aryl benzimidazole derivatives as non-cytotoxic potential dual inhibitors of α-glucosidase and urease enzymes. Bioorganic Chem. 2017, 72, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Taha, M.; Ullah, H.; Wadood, A.; Selvaraj, M.; Rab, A.; Sajid, M.; Shah, S.A.A.; Uddin, N.; Gollapalli, M. Synthesis of new arylhydrazide bearing Schiff bases/thiazolidinone: α-Amylase, urease activities and their molecular docking studies. Bioorganic Chem. 2019, 91, 103112. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.M.; Daneshfar, M.; Daneshfar, Z.; Iraji, A.; Samandari-Najafabad, A.; Faramarzi, M.A.; Mahdavi, M. Synthesis and characterization of 1-amidino-O-alkylureas metal complexes as α-glucosidase Inhibitors: Structure-activity relationship, molecular docking, and kinetic studies. J. Mol. Struct. 2022, 1250, 131726. [Google Scholar] [CrossRef]
- Azimi, F.; Azizian, H.; Najafi, M.; Hassanzadeh, F.; Sadeghi-Aliabadi, H.; Ghasemi, J.B.; Faramarzi, M.A.; Mojtabavi, S.; Larijani, B.; Saghaei, L.; et al. Design and synthesis of novel quinazolinone-pyrazole derivatives as potential α-glucosidase inhibitors: Structure-activity relationship, molecular modeling and kinetic study. Bioorganic Chem. 2021, 114, 105127. [Google Scholar] [CrossRef]
- Xie, H.X.; Zhang, J.; Li, Y.; Zhang, J.H.; Liu, S.K.; Zhang, J.; Zheng, H.; Hao, G.Z.; Zhu, K.K.; Jiang, C.S. Novel tetrahydrobenzo [b] thiophen-2-yl) urea derivatives as novel α-glucosidase inhibitors: Synthesis, kinetics study, molecular docking, and in vivo anti-hyperglycemic evaluation. Bioorganic Chem. 2021, 115, 105236. [Google Scholar] [CrossRef]
- Gani, R.S.; Kudva, A.K.; Timanagouda, K.; Mujawar, S.B.H.; Joshi, S.D.; Raghu, S.V. Synthesis of novel 5-(2,5-bis (2,2,2-trifluoroethoxy) phenyl)-1,3,4-oxadiazole-2-thiol derivatives as potential glucosidase inhibitors. Bioorganic Chem. 2021, 114, 105046. [Google Scholar] [CrossRef]
- Raghu, C.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary fibre for glycaemia control: Towards a mechanistic understanding. Bioact. Carbohydr. Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- Xiao, J.B.; Hogger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Kumar, L.; Lal, K.; Yadav, P.; Kumar, A.; Paul, A.K. Synthesis, characterization, α-glucosidase inhibition and molecular modeling studies of some pyrazoline-1H-1, 2, 3-triazole hybrids. J. Mol. Struct. 2020, 1216, 128253. [Google Scholar] [CrossRef]
- Ahmed, M.; Imran, M.; Muddassar, M.; Hussain, R.; Khan, M.U.; Ahmad, S.; Mehboob, M.Y.; Ashfaq, S. Benzenesulfonohydrazides inhibiting urease: Design, synthesis, their in vitro and in silico studies. J. Mol. Struct. 2020, 1220, 128740. [Google Scholar] [CrossRef]
- Shi, W.K.; Deng, R.C.; Wang, P.F.; Yue, Q.Q.; Liu, Q.; Ding, K.L.; Yang, M.H.; Zhang, H.Y.; Gong, S.H.; Deng, M.; et al. 3-Arylpropionylhydroxamic acid derivatives as Helicobacter pylori urease inhibitors: Synthesis, molecular docking and biological evaluation. Bioorganic Med. Chem. 2016, 24, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Khan, K.M.; Zehra, S.T.; Ahmed, R.; Shafiq, Z.; Bakht, S.M.; Yaqub, M.; Hussain, M.; de León, A.D.L.V.; Furtmann, N.; et al. Synthesis, biological evaluation and molecular docking of N-phenyl thiosemicarbazones as urease inhibitors. Bioorganic Chem. 2015, 61, 51–57. [Google Scholar] [CrossRef]
- Holter, H.; Linderstrøm-Lang, K. Micromethods and their application in the study of enzyme distribution in tissues and cells. Physiol. Rev. 1951, 31, 432–448. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Khan, A.; Shah, S.A.A.; Anwar, A.; Halim, S.A.; Fatmi, M.Q.; Imran, S.; Rahim, F.; Khan, K.M. Synthesis of novel derivatives of oxindole, their urease inhibition and molecular docking studies. Bioorganic Med. Chem. Lett. 2015, 25, 3285–3289. [Google Scholar] [CrossRef]
- Shehzad, M.T.; Khan, A.; Islam, M.; Halim, S.A.; Khiat, M.; Anwar, M.U.; Hussain, J.; Hameed, A.; Pasha, A.R.; Khan, F.A.; et al. Synthesis, characterization and molecular docking of some novel hydrazonothiazolines as urease inhibitors. Bioorganic Chem. 2020, 94, 103404. [Google Scholar] [CrossRef]
- Shehzad, M.T.; Khan, A.; Islam, M.; Hameed, A.; Khiat, M.; Halim, S.A.; Anwar, M.U.; Shah, S.R.; Hussain, J.; Csuk, R.; et al. Synthesis and urease inhibitory activity of 1, 4-benzodioxane-based thiosemicarbazones: Biochemical and computational approach. J. Mol. Struct. 2020, 1209, 127922. [Google Scholar] [CrossRef]
- Zonia, L.E.; Stebbins, N.E.; Polacco, J.C. Essential role of urease in germination of nitrogen-limited Arabidopsis thaliana seeds. Plant Physiol. 1995, 107, 1097–1103. [Google Scholar] [CrossRef]
- Zhengping, W.; Van Cleemput, O.; Demeyer, P.; Baert, L. Effect of urease inhibitors on urea hydrolysis and ammonia volatilization. Biol. Fertil. Soils 1991, 11, 43–47. [Google Scholar] [CrossRef]
- Li, W.Y.; Ni, W.W.; Ye, Y.X.; Fang, H.L.; Pan, X.M.; He, J.L.; Zhou, T.L.; Yi, J.; Liu, S.S.; Zhou, M.; et al. N-monoarylacetothioureas as potent urease inhibitors: Synthesis, SAR, and biological evaluation. J. Enzym. Inhib. Med. Chem. 2020, 35, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Aispuro-Pérez, A.; López-Ávalos, J.; García-Páez, F.; Montes-Avila, J.; Picos-Corrales, L.A.; Ochoa-Terán, A.; Bastidas, P.; Montaño, S.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Synthesis and molecular docking studies of imines as α-glucosidase and α-amylase inhibitors. Bioorganic Chem. 2020, 94, 103491. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khan, M.A.; Rahman, K.M.; Ahmad, I.; Ul-Haq, Z.; Khan, S.; Shafiq, Z. Development of sulfonamide-based Schiff bases targeting urease inhibition: Synthesis, characterization, inhibitory activity assessment, molecular docking and ADME studies. Bioorganic Chem. 2020, 102, 104057. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kitahara, F.; Nakamura, T.; Kojima, Y.; Fujino, M.A. Peptic ulcer in patients with diabetes mellitus. Nihon Rinsho. Jpn. J. Clin. Med. 2002, 60, 1580–1584. [Google Scholar]
- Tachecí, I.; Bures, J. Peptic ulcer disease in patients with diabetes mellitus. Vnitr. Lek. 2011, 57, 347–350. [Google Scholar]
- Rostom, S.A.; Badr, M.H.; Abd El Razik, H.A.; Ashour, H.M.; Abdel Wahab, A.E. Synthesis of some pyrazolines and pyrimidines derived from polymethoxy chalcones as anticancer and antimicrobial agents. Arch. Der Pharm. 2011, 344, 572–587. [Google Scholar] [CrossRef]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M. Unveiling a versatile heterocycle: Pyrazoline–a review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef]
- Chandra, T.; Garg, N.; Lata, S.; Saxena, K.K.; Kumar, A. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur. J. Med. Chem. 2010, 45, 1772–1776. [Google Scholar] [CrossRef]
- Karabacak, M.; Altıntop, M.D.; Çiftçi, H.İ.; Koga, R.; Otsuka, M.; Fujita, M.; Özdemir, A. Synthesis and evaluation of new pyrazoline derivatives as potential anticancer agents. Molecules 2015, 20, 19066–19084. [Google Scholar] [CrossRef]
- Kumar, K.A.; Govindaraju, M. Pyrazolines: Versatile molecules of synthetic and pharmaceutical applications-a review. Int. J. ChemTech Res 2015, 8, 313–322. [Google Scholar]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole ring—A biologically active scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Nehra, B.; Rulhania, S.; Jaswal, S.; Kumar, B.; Singh, G.; Monga, V. Recent advancements in the development of bioactive pyrazoline derivatives. Eur. J. Med. Chem. 2020, 205, 112666. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1, 3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M.; Eldebss, T.M.; El-Zahabi, H.S.; Yousef, M.H. Synthesis and antiviral activity of some new bis-1,3-thiazole derivatives. Eur. J. Med. Chem. 2015, 102, 266–276. [Google Scholar] [CrossRef]
- Gollapalli, M.; Taha, M.; Javid, M.T.; Almandil, N.B.; Rahim, F.; Wadood, A.; Mosaddik, A.; Ibrahim, M.; Alqahtani, M.A.; Bamarouf, Y.A. Synthesis of benzothiazole derivatives as a potent α-glucosidase inhibitor. Bioorganic Chem. 2019, 85, 33–48. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Partap, S.; Khisal, S.; Yar, M.S.; Mishra, R. Synthesis, anti-convulsant activity and molecular docking study of novel thiazole pyridazinone hybrid analogues. Bioorganic Chem. 2020, 99, 103584. [Google Scholar] [CrossRef]
- Jaishree, V.; Ramdas, N.; Sachin, J.; Ramesh, B. In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc. 2012, 16, 371–376. [Google Scholar] [CrossRef]
- Kasralikar, H.M.; Jadhavar, S.C.; Goswami, S.V.; Kaminwar, N.S.; Bhusare, S.R. Design, synthesis and molecular docking of pyrazolo [3, 4d] thiazole hybrids as potential anti-HIV-1 NNRT inhibitors. Bioorganic Chem. 2019, 86, 437–444. [Google Scholar] [CrossRef]
- Modrić, M.; Božičević, M.; Faraho, I.; Bosnar, M.; Škorić, I. Design, synthesis and biological evaluation of new 1, 3-thiazole derivatives as potential anti-inflammatory agents. J. Mol. Struct. 2021, 1239, 130526. [Google Scholar] [CrossRef]
- Kesari, C.; Rama, K.R.; Sedighi, K.; Stenvang, J.; Björkling, F.; Kankala, S.; Thota, N. Synthesis of thiazole linked chalcones and their pyrimidine analogues as anticancer agents. Synth. Commun. 2021, 51, 1406–1416. [Google Scholar] [CrossRef]
- Rana, M.; Arif, R.; Khan, F.I.; Maurya, V.; Singh, R.; Faizan, M.I.; Yasmeen, S.; Dar, S.H.; Alam, R.; Sahu, A.; et al. Pyrazoline analogs as potential anticancer agents and their apoptosis, molecular docking, MD simulation, DNA binding and antioxidant studies. Bioorganic Chem. 2021, 108, 104665. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Abas, B.İ.; Çevik, Ö.; Koçyiğit-Kaymakçıoğlu, B. Design, synthesis and biological evaluation of some new 2-Pyrazoline derivatives as potential anticancer agents. Bioorganic Chem. 2020, 102, 104063. [Google Scholar] [CrossRef] [PubMed]
- Ardiansah, B. Pharmaceutical importance of pyrazoline derivatives: A mini review. J. Pharm. Sci. Res. 2017, 9, 1958–1960. [Google Scholar]
- Matiadis, D.; Sagnou, M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int. J. Mol. Sci. 2020, 21, 5507. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; Sadiq, A.; Alsantali, R.I.; Mughal, E.U.; Alsharif, M.A.; Naeem, N.; Javid, A.; Al-Rooqi, M.M.; Chaudhry, G.E.S.; Ahmed, S.A. Synthesis and evaluation of 1, 3, 5-triaryl-2-pyrazoline derivatives as potent dual inhibitors of urease and α-glucosidase together with their cytotoxic, molecular modeling and drug-likeness studies. ACS-Omega 2022, 7, 3775–3795. [Google Scholar] [CrossRef] [PubMed]
- Zelelew, D.; Endale, M.; Melaku, Y.; Kedir, F.; Demissie, T.B.; Ombito, J.O.; Eswaramoorthy, R. Synthesis, Antibacterial, and Antioxidant Activities of Thiazolyl-Pyrazoline Schiff Base Hybrids: A Combined Experimental and Computational Study. J. Chem. 2022, 2022, 3717826. [Google Scholar] [CrossRef]
- Hawash, M.M.; Kahraman, D.C.; Eren, F.; Atalay, R.C.; Baytas, S.N. Synthesis and biological evaluation of novel pyrazolic chalcone derivatives as novel hepatocellular carcinoma therapeutics. Eur. J. Med. Chem. 2017, 129, 12–26. [Google Scholar] [CrossRef]
- Khan, Y.; Khan, S.; Rehman, W.; Hussain, R.; Maalik, A.; Ali, F.; Khan, M.U.; Sattar, A.; Assiri, M.A. Hybrid Molecules of Thiadiazole-Based Benzothioate and Benzenesulfonothioate: Synthesis, Structural Analysis, and Evaluation as Potential Inhibitors of Thymidine Phosphorylase and β-Glucuronidase through In Vitro and In Silico Approaches. J. Mol. Struct. 2023, 136439. [Google Scholar] [CrossRef]
- Hussain, R.; Rehman, W.; Rahim, F.; Khan, S.; Alanazi, A.S.; Alanazi, M.M.; Rasheed, L.; Khan, Y.; Shah, S.A.A.; Taha, M. Synthesis, in vitro thymidine phosphorylase inhibitory activity and molecular docking study of novel pyridine-derived bis-oxadiazole bearing bis-schiff base derivatives. Arab. J. Chem. 2023, 16, 104773. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Rahim, F.; Hussain, R.; Khan, Y.; Khan, M.S.; Iqbal, R.; Ali, B.; Albeshr, M.F. Synthesis, biological evaluation and molecular docking study of pyrimidine based thiazolidinone derivatives as potential anti-urease and anti-cancer agents. J. Saudi Chem. Soc. 2023, 27, 101688. [Google Scholar] [CrossRef]
- Zaman, K.; Rahim, F.; Taha, M.; Ullah, H.; Wadood, A.; Nawaz, M.; Khan, F.; Wahab, Z.; Shah, S.A.A.; Rehman, A.U.; et al. Synthesis, in vitro urease inhibitory potential and molecular docking study of Benzimidazole analogues. Bioorganic Chem. 2019, 89, 103024. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Iqbal, S.; Shah, M.; Rehman, W.; Khan, S.; Rasheed, L.; Rahim, F.; Dera, A.A.; Kehili, S.; Elkaeed, E.B.; et al. Synthesis of Novel Benzimidazole-Based Thiazole Derivatives as Multipotent Inhibitors of α-Amylase and α-Glucosidase: In Vitro Evaluation along with Molecular Docking Study. Molecules 2022, 27, 6457. [Google Scholar] [CrossRef]
- Hussain, R.; Rehman, W.; Rahim, F.; Khan, S.; Taha, M.; Khan, Y.; Sardar, A.; Khan, I.; Shah, S.A.A. Discovery of imidazopyridine derived oxadiazole-based thiourea derivatives as potential anti-diabetic agents: Synthesis, in vitro antioxidant screening and in silico molecular modeling approaches. J. Mol. Struct. 2023, 1293, 136185. [Google Scholar] [CrossRef]
- Saeedian Moghadam, E.; Al-Sadi, A.M.; Talebi, M.; Amanlou, M.; Amini, M.; and Abdel-Jalil, R. Novel benzimidazole derivatives; synthesis, bioactivity and molecular docking study as potent urease inhibitors. DARU J. Pharm. Sci. 2022, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]













| S. No. | R | α-Glucosidase IC50 (µM ±SEM) | Urease IC50(µM ± SEM) |
|---|---|---|---|
| 1 |  | 13.10 ± 0.40 | 41.50 ± 1.70 |
| 2 |  | 14.20 ± 0.10 | 37.20 ± 1.90 |
| 3 |  | 7.50 ± 0.10 | 33.20 ± 3.80 |
| 4 |  | 4.10 ± 0.30 | 23.30 ± 2.40 |
| 5 |  | 6.70 ±0.30 | 32.50 ± 2.10 |
| 6 |  | 2.50 ± 0.30 | 14.30 ± 3.90 |
| 7 |  | 3.20 ± 0.10 | 19.20 ± 0.10 |
| 8 |  | 13.10 ± 0.10 | 39.20 ± 0.60 |
| 9 |  | 4.40 ± 0.30 | 29.40 ± 0.10 |
| 10 |  | 8.90 ± 0.20 | 33.70 ± 0.10 |
| 11 |  | 3.80 ± 0.30 | 25.30 ± 0.20 |
| 12 |  | 3.50 ± 0.10 | 22.30 ± 0.80 |
| 13 |  | N.A * | N.A * |
| 14 |  | 3.40 ± 0.10 | 21.80 ± 2.90 |
| 15 |  | 17.50 ± 0.20 | 38.20 ± 0.50 |
| 16 |  | N.A * | N.A * |
| 17 |  | 6.80 ± 0.20 | 31.30 ± 0.40 |
| Standard drug Acarbose | 5.30 ± 0.30 | = | |
| Standard drug Thiourea | = | 31.40 ± 2.50 | |
| Active Analogues | Receptor | Types of INTERACTIONS | Distance (Ao) | Docking Score |
|---|---|---|---|---|
| Analogue-6 in α-glucosidase complex | GLY-A-116 | Pi-Pi T-shaped | 5.16 | −11.47 |
| TRP-A-231 | Pi-Pi T-shaped | 6.42 | ||
| LEU-A-286 | Pi-R | 5.72 | ||
| PHE-A-329 | Pi-Pi T-shaped | 6.81 | ||
| PHE-A-329 | Pi-Pi T-shaped | 7.57 | ||
| PHE-A-329 | Pi-Pi T-shaped | 5.53 | ||
| ALA-A-328 | Pi-R | 6.14 | ||
| ALA-A-328 | Pi-R | 4.61 | ||
| TRP-A-430 | Pi-R | 6.08 | ||
| GLY-A-78 | H-F | 5.40 | ||
| TRP-A-82 | Pi-R | 6.04 | ||
| TRP-A-82 | H-B | 5.16 | ||
| HIS-A-438 | H-F | 5.38 | ||
| TYR-A-332 | Pi-Pi T-shaped | 4.91 | ||
| TYR-A-332 | Pi-Pi T-shaped | 6.35 | ||
| Analogue-6 in Urease complex | TRP-A-84 | Pi-R | 5.87 | |
| PHE-A-330 | Pi-R | 5.81 | ||
| PHE-A-330 | Pi-Pi stacked | 5.69 | ||
| PHE-A-331 | Pi-S | 5.68 | ||
| TYR-A-70 | Pi-S | 6.30 | ||
| TRP-A-279 | Pi-S | 6.53 | ||
| TRP-A-279 | Pi-R | 4.44 | ||
| TRP-A-279 | Pi-Pi stacked | 5.20 | ||
| ASP-A-276 | H-F | 6.13 | ||
| ASN-A-280 | H-F | 4.97 | ||
| ASN-A-280 | H-F | 4.65 | ||
| Analogue-7 in α-glucosidase complex | GLY-A-116 | Pi-Pi stacked | 4.82 | |
| LEU-A-286 | Pi-R | 5.88 | ||
| PHE-A-329 | Pi-Pi stacked | 6.92 | ||
| THR-A-120 | H-B | 4.33 | ||
| GLU-A-197 | H-F | 3.27 | ||
| TYR-A-128 | H-B | 6.85 | ||
| Analogue-7 in Urease complex | PHE-A-330 | Pi-Pi stacked | 5.62 | −10.17 |
| PHE-A-330 | Pi-R | 5.82 | ||
| TRP-A-279 | Pi-Pi stacked | 6.52 | ||
| TRP-A-279 | Pi-Pi stacked | 5.75 | ||
| TRP-A-279 | Pi-Pi stacked | 4.91 | ||
| TYR-A-70 | Pi-S | 5.88 | ||
| TYR-A-70 | Pi-Pi stacked | 6.72 | ||
| TYR-A-70 | Pi-Pi stacked | 5.79 | ||
| TRP-A-84 | Pi-R | 5.32 | ||
| ASP-A-285 | H-F | 4.84 | ||
| TYR-A-334 | Pi-Pi stacked | 4.73 | ||
| PHE-A-331 | Pi-S | 4.45 | ||
| Analogue-12 in α-glucosidase complex | PHE-A-329 | Pi-S | 6.72 | −10.01 |
| PHE-A-329 | Pi-Pi stacked | 7.23 | ||
| PHE-A-329 | Pi-Pi stacked | 6.93 | ||
| TRP-A-331 | Pi-Pi stacked | 6.40 | ||
| LEU-A-286 | Pi-R | 5.89 | ||
| TRP-A-82 | Pi-Pi stacked | 4.62 | ||
| TYR-A-128 | H-B | 6.52 | ||
| GLU-A-197 | H-F | 4.57 | ||
| Analogue-12 in Urease complex | PHE-A-330 | Pi-R | 3.90 | −9.98 |
| PHE-A-330 | Pi-Pi stacked | 4.73 | ||
| TYR-A-334 | Pi-Sigma | 4.48 | ||
| TYR-A-334 | Pi-Pi stacked | 4.31 | ||
| TYR-A-334 | Pi-Pi stacked | 4.30 | ||
| TYR-A-70 | Pi-S | 5.60 | ||
| ASP-A-285 | H-F | 4.65 | ||
| PHE-A-331 | Pi-S | 4.40 | ||
| PHE-A-331 | Pi-R | 5.27 | ||
| TRP-A-279 | Pi-Pi stacked | 6.73 | ||
| TRP-A-279 | Pi-S | 7.59 | ||
| HIS-A-440 | Pi-R | 4.29 | ||
| Analogue-14 in α-glucosidase complex | GLY-A-116 | Pi-Pi stacked | 4.90 | −9.27 |
| TRP-A-231 | Pi-Pi stacked | 5.94 | ||
| LEU-A-286 | Pi-R | 4.94 | ||
| ASP-A-70 | H-B | 7.13 | ||
| ASP-A-70 | Pi-anion | 7.47 | ||
| MET-A-437 | Pi-R | 5.93 | ||
| TYR-A-440 | Pi-R | 6.15 | ||
| PHE-A-329 | Pi-Pi stacked | 6.89 | ||
| PHE-A-329 | Pi-anion | 4.71 | ||
| PHE-A-329 | Pi-Pi stacked | 5.45 | ||
| PHE-A-329 | Pi-sigma | 3.49 | ||
| TRP-A-82 | Pi-R | 5.43 | ||
| ALA-A-328 | Pi-R | 6.05 | ||
| TYR-A-332 | Pi-Pi stacked | 5.54 | ||
| TYR-A-332 | Pi-Pi stacked | 6.40 | ||
| Analogue-14 in Urease complex | PHE-A-330 | Pi-S | 5.65 | −8.76 |
| PHE-A-331 | Pi-R | 5.66 | ||
| TYR-A-70 | Pi-Pi stacked | 6.39 | ||
| TRP-A-279 | Pi-R | 6.59 | ||
| TRP-A-279 | Pi-Pi stacked | 4.24 | ||
| TRP-A-279 | Pi-Pi stacked | 4.78 | ||
| Standard Acarbose | ASP-A-422 | HB | 4.50 | |
| ASP-A-630 | UNFAVORABLE AA | 4.74 | ||
| ALA-A-602 | HB | 5.29 | ||
| GLU-A-603 | HB | 4.08 | ||
| SER-A-505 | HB | 3.72 | ||
| SER-A-505 | CH | 3.31 | ||
| SER-A-505 | HB | 3.53 | ||
| ASN-A-496 | HB | 4.81 | ||
| ILE-A-233 | HB | 3.71 | ||
| Standard Thiourea | HIS-C-315 | PiS | 7.23 | |
| THR-C-301 | HB | 2.73 | ||
| THR-C-304 | HB | 4.28 | ||
| GLU-C-348 | HB | 5.26 | ||
| ALA-C-279 | Unfavourable-DD | 2.78 | ||
| GLU-C-277 | HB | 3.76 | ||
| GLY-C-280 | Unfavourable-DD | 3.58 | ||
| GLY-C-280 | HB | 4.14 | ||
| GLY-C-278 | Unfavourable-DD | 3.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Y.; Khan, S.; Hussain, R.; Maalik, A.; Rehman, W.; Attwa, M.W.; Masood, R.; Darwish, H.W.; Ghabbour, H.A. The Synthesis, In Vitro Bio-Evaluation, and In Silico Molecular Docking Studies of Pyrazoline–Thiazole Hybrid Analogues as Promising Anti-α-Glucosidase and Anti-Urease Agents. Pharmaceuticals 2023, 16, 1650. https://doi.org/10.3390/ph16121650
Khan Y, Khan S, Hussain R, Maalik A, Rehman W, Attwa MW, Masood R, Darwish HW, Ghabbour HA. The Synthesis, In Vitro Bio-Evaluation, and In Silico Molecular Docking Studies of Pyrazoline–Thiazole Hybrid Analogues as Promising Anti-α-Glucosidase and Anti-Urease Agents. Pharmaceuticals. 2023; 16(12):1650. https://doi.org/10.3390/ph16121650
Chicago/Turabian StyleKhan, Yousaf, Shoaib Khan, Rafaqat Hussain, Aneela Maalik, Wajid Rehman, Mohamed W. Attwa, Rafia Masood, Hany W. Darwish, and Hazem A. Ghabbour. 2023. "The Synthesis, In Vitro Bio-Evaluation, and In Silico Molecular Docking Studies of Pyrazoline–Thiazole Hybrid Analogues as Promising Anti-α-Glucosidase and Anti-Urease Agents" Pharmaceuticals 16, no. 12: 1650. https://doi.org/10.3390/ph16121650
APA StyleKhan, Y., Khan, S., Hussain, R., Maalik, A., Rehman, W., Attwa, M. W., Masood, R., Darwish, H. W., & Ghabbour, H. A. (2023). The Synthesis, In Vitro Bio-Evaluation, and In Silico Molecular Docking Studies of Pyrazoline–Thiazole Hybrid Analogues as Promising Anti-α-Glucosidase and Anti-Urease Agents. Pharmaceuticals, 16(12), 1650. https://doi.org/10.3390/ph16121650