Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A
Abstract
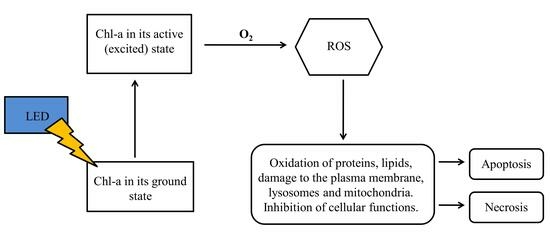
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Temperature Tsol-gel Properties of Formulations
2.2. In Vitro Bioadhesion Assay
2.3. Characterization by ATR-FTIR
2.4. Characterization by SEM
2.5. Biological Assay
3. Materials and Methods
3.1. Materials and Reagents
3.2. Formulation of HGs and Incorporation of Chl-A in HGs
3.3. Evaluation of the Sol-Gel Transition Temperature (Tsol-gel) of HG-CS-P407
3.4. Evaluation of Tsol-gel Time
3.5. In Vitro Bioadhesion Assay
3.6. Attenuated Total Reflectance with Fourier Transform Infrared Spectroscopy (ATR/FTIR)
3.7. Scanning Electron Microscopy (SEM)
3.8. Cell Culture Conditions
3.9. PDT Mediated by HG-CS-P407 Containing Chl-A
3.10. Cell Viability Assays
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jhappan, C.; Noonan, F.P.; Merlino, G. Ultraviolet radiation and cutaneous malignant melanoma. Oncogene 2003, 22, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. The Global Cancer Observatory. 2020. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed on 29 January 2021).
- Chen, K.G.; Leapman, R.D.; Zhang, G.; Lai, B.; Valencia, J.C.; Cardarelli, C.O.; Vieira, W.D.; Hearing, V.J.; Gottesman, M.M. Influence of Melanosome Dynamics on Melanoma Drug Sensitivity. J. Natl. Cancer Inst. 2009, 101, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; da Silva Costa, N.R.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Marks, M.S. The Dark Side of Lysosome-Related Organelles: Specialization of the Endocytic Pathway for Melanosome Biogenesis. Traffic 2002, 3, 237–248. [Google Scholar] [CrossRef]
- Liu, M.-H.; Li, Y.-F.; Chen, B.-H. Preparation of Chlorophyll Nanoemulsion from Pomelo Leaves and Its Inhibition Effect on Melanoma Cells A375. Plants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Grainger, D.W. Connecting drug delivery reality to smart materials design. Int. J. Pharm. 2013, 454, 521–524. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C 2016, 66, 178–184. [Google Scholar] [CrossRef]
- da Silva Souza Campanholi, K.; Braga, G.; da Silva, J.B.; da Rocha, N.L.; de Francisco, L.M.B.; de Oliveira, L.; Bruschi, M.L.; de Castro-Hoshino, L.V.; Sato, F.; Hioka, N.; et al. Biomedical Platform Development of a Chlorophyll-Based Extract for Topic Photodynamic Therapy: Mechanical and Spectroscopic Properties. Langmuir 2018, 34, 8230–8244. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Xia, F.; Zhang, X. Gelatin/polyacrylamide ionic conductive hydrogel with skin temperature-triggered adhesion for human motion sensing and body heat harvesting. Nano Energy 2022, 104, 107977. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Tuğcu-Demiṙöz, F. Development of in situ poloxamer-chitosan hydrogels for vaginal drug delivery of benzydamine hydrochloride: Textural, mucoadhesive and in vitro release properties. Marmara Pharm. J. 2017, 21, 762–770. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Kaleem, M.; Dalhat, M.H. Thermosensitive Hydrogels Loaded with Resveratrol Nanoemulsion: Formulation Optimization by Central Composite Design and Evaluation in MCF-7 Human Breast Cancer Cell Lines. Gels 2022, 8, 450. [Google Scholar] [CrossRef]
- Argenta, D.F.; da Costa Bernardo, B.; Chamorro, A.F.; Matos, P.R.; Caon, T. Thermosensitive hydrogels for vaginal delivery of secnidazole as an approach to overcome the systemic side-effects of oral preparations. Eur. J. Pharm. Sci. 2021, 159, 105722. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 89. [Google Scholar] [CrossRef]
- Shin, S.-C.; Lee, J.-W.; Yang, K.-H.; Lee, C.H. Preparation and evaluation of bioadhesive benzocaine gels for enhanced local anesthetic effects. Int. J. Pharm. 2003, 260, 77–81. [Google Scholar] [CrossRef]
- Cafaggi, S.; Leardi, R.; Parodi, B.; Caviglioli, G.; Russo, E.; Bignardi, G. Preparation and evaluation of a chitosan salt–poloxamer 407 based matrix for buccal drug delivery. J. Control. Release 2005, 102, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Mittal, K.L. (Eds.) Handbook of Adhesive Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Karolewicz, B.; Gajda, M.; Górniak, A.; Owczarek, A.; Mucha, I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J. Therm. Anal. Calorim. 2017, 130, 383–390. [Google Scholar] [CrossRef]
- Vino, A.B.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848. Asian Pac. J. Trop. Biomed. 2012, 2, S334–S341. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Shimanouchi, T. Tables of Molecular Vibrational Frequencies; National Bureau of Standards: Washington, DC, USA, 1972. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introdução à Espectroscopia: Tradução da 4ª Ediçãonorte-Americana; Cengage-Learning: São Paulo, Brazil, 2010. [Google Scholar]
- Vyas, V.; Sancheti, P.; Karekar, P.; Shah, M.; Pore, Y. Physicochemical characterization of solid dispersion systems of tadalafil with poloxamer 407. Acta Pharm. 2009, 59, 453–461. [Google Scholar] [CrossRef]
- Kim, S.H.; Chu, C.C. Synthesis and characterization of dextran–methacrylate hydrogels and structural study by SEM. J. Biomed. Mater. Res. 2000, 49, 517–527. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Sosnik, A.; Cohn, D. Ethoxysilane-capped PEO–PPO–PEO triblocks: A new family of reverse thermo-responsive polymers. Biomaterials 2004, 25, 2851–2858. [Google Scholar] [CrossRef]
- Shah, E.T.; Mesrati, H.A.O.; Aldallal, U.J.; Abdulwahab, F.; Henari, F.Z.; Safrany, S.T. Assessing the photodynamic therapeutic effects of 5-aminolevulinic acid on human colon cancer cells using light-emitting diodes. World Acad. Sci. J. 2021, 3, 43. [Google Scholar] [CrossRef]
- Morais, J.A.V.; Almeida, L.R.; Rodrigues, M.C.; Azevedo, R.B.; Muehlmann, L.A. The induction of immunogenic cell death by photodynamic therapy in B16F10 cells in vitro is effected by the concentration of the photosensitizer. Photodiagnosis Photodyn. Ther. 2021, 35, 102392. [Google Scholar] [CrossRef]
- da Hora Machado, A.E. Terapia fotodinâmica: Princípios, potencial de aplicação e perspectivas. Quim. Nova. 2000, 23, 237–243. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Cui, S.; Chen, H.; Zhu, H.; Tian, J.; Chi, X.; Qian, Z.; Achilefu, S.; Gu, Y. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Gray, J.; Fullarton, G. The current role of photodynamic therapy in oesophageal dysplasia and cancer. Photodiagnosis Photodyn. Ther. 2007, 4, 151–159. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef]
- Giovagnoli, S.; Tsai, T.; DeLuca, P.P. Formulation and Release Behavior of Doxycycline–Alginate Hydrogel Microparticles Embedded into Pluronic F127 Thermogels as a Potential New Vehicle for Doxycycline Intradermal Sustained Delivery. AAPS PharmSciTech 2010, 11, 212–220. [Google Scholar] [CrossRef]
- Hani, U.; Bhat, R.S.; Shiva-ku-mar, H.G. Formulation design and evaluation of metronidazole microspheres in a bioadhesive gel for local therapy of vaginal candidiasis. Lat. Am. J. Pharm. 2011, 30, 161. [Google Scholar]
- Dick, I.P.; Scott, R.C. Pig Ear Skin as an In-vitro Model for Human Skin Permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Lv, H.-F.; Lu, C.-T.; Chen, L.-J.; Lin, M.; Zhang, M.; Jiang, X.; Shen, X.-T.; Jin, R.-R.; Cai, J.; et al. Evaluation of a Novel Thermosensitive Heparin-Poloxamer Hydrogel for Improving Vascular Anastomosis Quality and Safety in a Rabbit Model. PLoS ONE 2013, 8, e73178. [Google Scholar] [CrossRef]
- Gerola, A.P.; Santana, A.; França, P.B.; Tsubone, T.M.; de Oliveira, H.P.M.; Caetano, W.; Kimura, E.; Hioka, N. Effects of Metal and the Phytyl Chain on Chlorophyll Derivatives: Physicochemical Evaluation for Photodynamic Inactivation of Microorganisms. Photochem. Photobiol. 2011, 87, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]






| Sample | Viscosity at 5 °C (Pa.s) | Viscosity at 20 °C (Pa.s) | Viscosity at 34 °C (Pa.s) | Transition Temperature (°C) |
|---|---|---|---|---|
| PL14 | 0.02 | 0.01 | 0.02 | 24.50 |
| PL14CS | 0.69 | 0.58 | 1.63 | 18.10 |
| PL16 | 0.02 | 0.04 | 13.25 | 19.0–31.74 |
| PL16CS | 0.81 | 1.01 | 6.21 | 16.73–34.46 |
| PL18 | 0.03 | 0.04 | 21.31 | 18.6–26.70 |
| PL18CS | 1.07 | 1.64 | 29.44 | 15.81–24.20 |
| PL20 | 0.04 | 0.11 | 34.28 | 14.43–24.90 |
| PL20CS | 1.75 | 10.59 | 35.11 | 14.44–22.64 |
| Identification | CS (%) | P407 (%) | Time (s) |
|---|---|---|---|
| V1 | 1.0 | 14 | 10.24 |
| V2 | 1.0 | 16 | 10.20 |
| V3 | 1.0 | 18 | 10.23 |
| V4 | 1.0 | 20 | n/a |
| V1.A | n/a | 14 | 30.12 |
| V2.B | n/a | 16 | n/a |
| V3.C | n/a | 18 | n/a |
| V4.D | n/a | 20 | n/a |
| Identification | CS (%) | P407 (%) |
|---|---|---|
| V1 | 1.0 | 14 |
| V2 | 1.0 | 16 |
| V3 | 1.0 | 18 |
| V4 | 1.0 | 20 |
| V1.A | n/a | 14 |
| V2.B | n/a | 16 |
| V3.C | n/a | 18 |
| V4.D | n/a | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.L.; da Silva, P.B.; Fonseca-Santos, B.; Báo, S.N.; Chorilli, M.; de Souza, P.E.N.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A. Pharmaceuticals 2023, 16, 1659. https://doi.org/10.3390/ph16121659
Araújo JL, da Silva PB, Fonseca-Santos B, Báo SN, Chorilli M, de Souza PEN, Muehlmann LA, Azevedo RB. Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A. Pharmaceuticals. 2023; 16(12):1659. https://doi.org/10.3390/ph16121659
Chicago/Turabian StyleAraújo, Joabe Lima, Patrícia Bento da Silva, Bruno Fonseca-Santos, Sônia Nair Báo, Marlus Chorilli, Paulo Eduardo Narcizo de Souza, Luis Alexandre Muehlmann, and Ricardo Bentes Azevedo. 2023. "Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A" Pharmaceuticals 16, no. 12: 1659. https://doi.org/10.3390/ph16121659
APA StyleAraújo, J. L., da Silva, P. B., Fonseca-Santos, B., Báo, S. N., Chorilli, M., de Souza, P. E. N., Muehlmann, L. A., & Azevedo, R. B. (2023). Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A. Pharmaceuticals, 16(12), 1659. https://doi.org/10.3390/ph16121659