Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes
Abstract
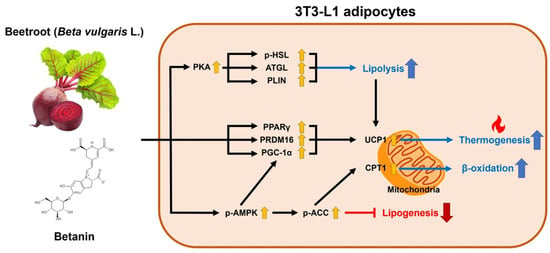
:1. Introduction
2. Results
2.1. Effects of BT on Cell Viability and Lipid Accumulation in 3T3-L1 Adipocytes
2.2. Effects of BT on Browning Factors in 3T3-L1 Adipocytes
2.3. Effects of BT on Mitochondrial and UCP1 Activation in 3T3-L1 Adipocytes
2.4. Effects of BT on Lipid Metabolism in 3T3-L1 Adipocytes
2.5. Effects of BT on UCP1 Expression via Activation of the AMPK Signaling Pathway in 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Differentiation
4.3. MTT Assay
4.4. Oil-Red-O Staining
4.5. Mitochondrial Analysis and Immunofluorescence
4.6. Western Blotting Analysis
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Payab, M.; Hasani-Ranjbar, S.; Baeeri, M.; Rahimifard, M.; Arjmand, B.; Haghi-Aminjan, H.; Abdollahi, M.; Larijani, B. Development of a Novel Anti-Obesity Compound with Inhibiting Properties on the Lipid Accumulation in 3T3-L1 Adipocytes. Iran. Biomed. J. 2020, 24, 155–163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Consultation on Obesity. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Shi, Y.; Burn, P. Lipid Metabolic Enzymes: Emerging Drug Targets for the Treatment of Obesity. Nat. Rev. Drug Discov. 2004, 3, 695–710. [Google Scholar] [CrossRef]
- Wadden, T.A.; Berkowitz, R.I.; Womble, L.G.; Sarwer, D.B.; Phelan, S.; Cato, R.K.; Hesson, L.A.; Osei, S.Y.; Kaplan, R.; Stunkard, A.J. Randomized Trial of Lifestyle Modification and Pharmacotherapy for Obesity. N. Engl. J. Med. 2005, 353, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.M.Y.; Cheung, T.T.; Samaranayake, N.R. Safety of Antiobesity Drugs. Ther. Adv. Drug Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-Obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef]
- Choi, J.H.; Yun, J.W. Chrysin Induces Brown Fat–like Phenotype and Enhances Lipid Metabolism in 3T3-L1 Adipocytes. Nutrition 2016, 32, 1002–1010. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.W.; Yu, R.; Yun, J.W. Monoterpene Phenolic Compound Thymol Promotes Browning of 3T3-L1 Adipocytes. Eur. J. Nutr. 2017, 56, 2329–2341. [Google Scholar] [CrossRef]
- Kang, N.H.; Mukherjee, S.; Yun, J.W. Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes. Nutrients 2019, 11, 577. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, W.; Yin, X.; Zheng, X.; Li, Q.; Zhang, H.; Zheng, L.; Feng, X. Resveratrol-Induced Brown Fat-like Phenotype in 3T3-L1 Adipocytes Partly via mTOR Pathway. Food Nutr. Res. 2020, 64, 3656. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Tseng, Y.-H. Brown Fat Fuel Utilization and Thermogenesis. Trends Endocrinol. Metab. 2014, 25, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Heo, C.U.; Song, Y.-H.; Lee, K.; Choi, C.-I. Naringin Promotes Fat Browning Mediated by UCP1 Activation via the AMPK Signaling Pathway in 3T3-L1 Adipocytes. Arch. Pharm. Res. 2023, 46, 192–205. [Google Scholar] [CrossRef]
- Park, A.; Kim, W.K.; Bae, K.-H. Distinction of White, Beige and Brown Adipocytes Derived from Mesenchymal Stem Cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Spiegelman, B.M. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015, 64, 2346–2351. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Münzberg, H.; Zhang, Z.-Y.; Kahn, B.B.; et al. Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Lin, J.Z.; Aprahamian, T.R.; Farmer, S.R. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1+ Adipocytes. Cell Metab. 2016, 24, 835–847. [Google Scholar] [CrossRef]
- Hussain, M.F.; Roesler, A.; Kazak, L. Regulation of Adipocyte Thermogenesis: Mechanisms Controlling Obesity. FEBS J. 2020, 287, 3370–3385. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D. Uncoupling Protein 1 of Brown Adipocytes, the Only Uncoupler: A Historical Perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, A.-C.; Paz, H.A.; Wankhade, U.D. Beige Adipose Tissue Identification and Marker Specificity—Overview. Front. Endocrinol. 2021, 12, 599134. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological Effects of Red Beetroot and Betalains: A Review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Al-Dosari, M.; Alqasoumi, S.; Al-Yahya, M.; Ansari, M.N. Effect of beta vulgaris l. On cholesterol rich diet-induced hypercholesterolemia in rats. Farmacia 2011, 59, 669–678. [Google Scholar]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Claussnitzer, M.; Dankel, S.N.; Kim, K.-H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. 2015, 373, 895–907. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and Beige Fat in Humans: Thermogenic Adipocytes That Control Energy and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and Nutritional Agents Promoting Browning of White Adipose Tissue. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2013, 1831, 969–985. [Google Scholar] [CrossRef]
- Kooijman, S.; Heuvel, J.K.V.D.; Rensen, P.C.N. Neuronal Control of Brown Fat Activity. Trends Endocrinol. Metab. 2015, 26, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Medvedev, A.V.; Daniel, K.W.; Collins, S. β-Adrenergic Activation of P38 MAP Kinase in Adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires P38 MAP Kinase. J. Biol. Chem. 2001, 276, 27077–27082. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, M.-H.; Hung, W.-L.; Tung, Y.-C.; Ho, C.-T. From White to Beige Adipocytes: Therapeutic Potential of Dietary Molecules against Obesity and Their Molecular Mechanisms. Food Funct. 2019, 10, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα Induces Adipogenesis through PPARγ: A Unified Pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Cianciosi, D.; Ansary, J.; Mezzetti, B.; Bompadre, S.; Quiles, J.L.; Giampieri, F.; Battino, M. Strawberry (Fragaria × Ananassa Cv. Romina) Methanolic Extract Promotes Browning in 3T3-L1 Cells. Food Funct. 2020, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.A.; Sun, L. Turning WAT into BAT: A Review on Regulators Controlling the Browning of White Adipocytes. Biosci. Rep. 2013, 33, e00065. [Google Scholar] [CrossRef] [PubMed]
- Emont, M.P.; Kim, D.; Wu, J. Development, Activation, and Therapeutic Potential of Thermogenic Adipocytes. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2019, 1864, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARγ Agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef]
- O’Neill, H.M.; Holloway, G.P.; Steinberg, G.R. AMPK Regulation of Fatty Acid Metabolism and Mitochondrial Biogenesis: Implications for Obesity. Mol. Cell Endocrinol. 2013, 366, 135–151. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Morais, V.A. Mitochondrial Biogenesis in Neurons: How and Where. Int. J. Mol. Sci. 2021, 22, 13059. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Fuda, H.; Tsukui, T.; Chiba, H.; Hui, S.-P. Discovery of Eicosapentaenoic Acid Esters of Hydroxy Fatty Acids as Potent Nrf2 Activators. Antioxidants 2020, 9, 397. [Google Scholar] [CrossRef]
- Li, Y.; Fromme, T.; Schweizer, S.; Schöttl, T.; Klingenspor, M. Taking Control over Intracellular Fatty Acid Levels Is Essential for the Analysis of Thermogenic Function in Cultured Primary Brown and Brite/Beige Adipocytes. EMBO Rep. 2014, 15, 1069–1076. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis–A Highly Regulated Multi-Enzyme Complex Mediates the Catabolism of Cellular Fat Stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, M.W.; Rönnstrand, L.; Wernstedt, C.; Degerman, E.; Holm, C. Identification of Novel Phosphorylation Sites in Hormone-Sensitive Lipase That Are Phosphorylated in Response to Isoproterenol and Govern Activation Properties In Vitro. J. Biol. Chem. 1998, 273, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Dominguez, M.; Sebastián, D.; Fucho, R.; Weber, M.; Mir, J.F.; García-Casarrubios, E.; Obregón, M.J.; Zorzano, A.; Valverde, Á.M.; Serra, D.; et al. Carnitine Palmitoyltransferase 1 Increases Lipolysis, UCP1 Protein Expression and Mitochondrial Activity in Brown Adipocytes. PLoS ONE 2016, 11, e0159399. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M.; Li, L.O.; Wu, P.-C.; Koves, T.R.; Ilkayeva, O.; Stevens, R.D.; Watkins, S.M.; Muoio, D.M.; Coleman, R.A. Adipose Acyl-CoA Synthetase-1 Directs Fatty Acids toward β-Oxidation and Is Required for Cold Thermogenesis. Cell Metab. 2010, 12, 53–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Xiang, J.; Zhou, J.; Cao, H.; Che, Q.; Bai, Y.; Guo, J.; Su, Z. Non-Shivering Thermogenesis Signalling Regulation and Potential Therapeutic Applications of Brown Adipose Tissue. Int. J. Biol. Sci. 2021, 17, 2853–2870. [Google Scholar] [CrossRef]






| Gene | Forward | Reverse |
|---|---|---|
| Aco1 | ATCCAGACTTCCAACATFAG | AACCACATGATTTCTTCAGG |
| Atgl | TTCACCATCCGCTTGTTGGAG | AGATGGTCACCCAATTTCCTC |
| Cd137 | GGTCTGTGCTTAAGACCGGG | TCTTAATAGCTGGTCCTCCCTC |
| Cidea | CGGGAATAGCCAGAGTCACC | TGTGCATCGGATGTCGTAGG |
| Cited1 | AACCTTGGAGTGAAGGATCGC | GTAGGAGAGCCTATTGGAGATGT |
| Cox4 | TGACGGCCTTGGACGG | CGATCAGCGTAAGTGGGGA |
| Cpt1 | GTGTTGGAGGTGACAGACTT | CACTTTCTCTTTCCACAAGG |
| Fgf21 | CGTCTGCCTCAGAAGGACTC | TCTACCATGCTCAGGGGGTC |
| Hsl | TGTCGTAGTGGCCGTTCTGA | CACACTGAGGCCTGTCTCGTT |
| Nrf1 | GCTAATGGCCTGGTCCAGAT | CTGCGCTGTCCGATATCCTG |
| Pgc1a | ATGTGCAGCCAAGACTCTGTA | CGCTACACCACTTCAATCCAC |
| Plin1 | GCAAGAAGAGCTGAGCAGAC | AATCTGCCCACGAGAAAGGA |
| Pparg | CAAGAATACCAAAGTGCGATCAA | GAGCTGGGTCTTTTCAGAATAATAAG |
| Prdm16 | GATGGGAGATGCTGACGGAT | TGATCTGACACATGGCGAGG |
| Tbx1 | AGCGAGGCGGAAGGGA | CCTGGTGACTGTGCTGAAGT |
| Tfam | ATGTGGAGCGTGCTAAAAGC | GGATAGCTACCCATGCTGGAA |
| Tmem26 | CCATGGAAACCAGTATTGCAGC | ATTGGTGGCTCTGTGGGATG |
| Ucp1 | CCTGCCTCTCTCGGAAACAA | GTAGCGGGGTTTGATCCCAT |
| GAPDH | TTGTTGCCATCAACGACCCC | GCCGTTGAATTTGCCGTGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Choi, S.M.; Lim, S.H.; Choi, C.-I. Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals 2023, 16, 1727. https://doi.org/10.3390/ph16121727
Lee HS, Choi SM, Lim SH, Choi C-I. Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals. 2023; 16(12):1727. https://doi.org/10.3390/ph16121727
Chicago/Turabian StyleLee, Ho Seon, Seung Min Choi, Sung Ho Lim, and Chang-Ik Choi. 2023. "Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes" Pharmaceuticals 16, no. 12: 1727. https://doi.org/10.3390/ph16121727
APA StyleLee, H. S., Choi, S. M., Lim, S. H., & Choi, C. -I. (2023). Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals, 16(12), 1727. https://doi.org/10.3390/ph16121727