Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review
Abstract
:1. Introduction
2. An Overview of Wounds and Their Consequences
2.1. Chronic and Acute Wound Healing
2.2. Progression of Healing a Wound
2.2.1. Hemostasis
2.2.2. Inflammation
2.2.3. Proliferation
2.2.4. Remodeling
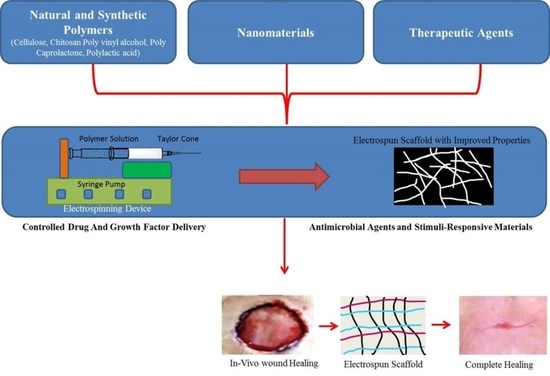
3. Electrospinning Is an Emerging Way to Create Polymer Nanofibrous Structures for Aiding in Wound Healing
4. The Influence of Different Factors on Electrospinning Is Investigated
4.1. Effect of Applied Voltage
4.2. Effect of Solution Flow Rate
4.3. Influence of the Needle-to-Collector Distance and Needle Diameter
4.4. Effects of Polymer Concentration and Solution Viscosity
4.5. Effect of Electrical Conductivity
4.6. Role of Solvent in Electrospinning
4.7. Effect of Humidity and Temperature
| Parameters | Effect on Fiber Morphology | References |
|---|---|---|
| Viscosity | A thicker fiber diameter is caused by a thicker consistency of the liquid. There is no any continuous fiber formation if the viscosity is very low, and it is challenging to expel the jet from the needle tip if it is too high. | [47] |
| Polymer concentration | Increase in fiber diameter with an increase in concentration. | [48] |
| Molecular weight of polymer | Reduction in the number of beads and droplets with an increase in molecular weight. | [49] |
| Electrical Conductivity | Decrease in fiber diameter with an increase in conductivity. | [50] |
| Applied voltage | Decrease in fiber diameter with an increase in voltage. | [51] |
| Distance between tip and collector | Generation of beads with too small and too large distances, a minimum distance required for uniform fibers. | [52] |
| Feed rate/Flow rate | Decrease in fiber diameter with a decrease in flow rate, generation of beads with too high flow rate. | [53] |
| Humidity | High humidity results in circular pores on the fibers. | [54] |
| Temperature | Increase in temperature results in a decrease in fiber diameter. | [54] |
5. Wound Dressings with Multiple Functions
5.1. Antibacterial Activity of Electrospun Nanofibers for Wound Dressing
| Electrospun Material | Nanoparticle | Bacterial Species | Composite Nanofiber Diameter | Electrospinning Parameter | Reference |
|---|---|---|---|---|---|
| The polyethylene oxide/Graphene oxide was electrospun with peppermint oil | CeO2 | S. aureus and E. coli | 310–365 nm | Electrospinning was conducted 15 cm away from the source and with a voltage of 15 kV, while the feeding rate remained at 1 mL per hour. | [63] |
| Nanofibrous scaffolds from Gum Arabic, polycaprolactone, and polyvinyl alcohol | Ag (10–100 nm) | S. aureus, P. aeruginosa, E. coli, and fungus Candida albicans | 150–250 nm | A 10 mL syringe that held the ready-fabricated polymeric solution was filled with it, and a syringe pump was used to deliver it at a flow rate of 0.5 mL/h. The voltage that was used was 18 kV, and the distance between the tip and the collector was 150 mm. | [64] |
| Electrospun Chitosan/Gelatin | Fe3O4 | S. aureus and E. coli | 307–435 nm | The following electrospinning parameters were set: 0.8 mL/h feeding rate; 15 kV voltage; and 15 cm distance between the needle tip and collector. | [65] |
| Polyethylene Oxide/Carboxymethyl Chitosan Nanofibers | Ag (12–18 nm) | S. aureus, P. aeruginosa, E. coli, and fungus Candida albicans | 50–300 nm | The composite fiber membranes were fabricated as follows: 40% relative humidity, a 20 cm span between needle-to-collector, and a 20 kV applied spinning voltage. | [58] |
| Polyvinyl alcohol–chitosan composite electrospun nanofibers | Ag | S. aureus, P. aeruginosa, and E. coli | 150 nm | At room temperature and relative humidity of 45.5%, the electrospinning procedure was completed. The collector was placed 15 cm from the needle tip, voltage (15 kV) was used. A single syringe piston pump was used to regulate the solution feed rate at 0.5 mL/min. | [61] |
| Electrospun chitosan nanofibers | Ag | S. aureus, and P. aeruginosa | The average fiber diameters of CTS and CTS/AgNPs nanofibers were 460 ± 80 nm, 126 ± 28 nm, 238 ± 46 nm, 343 337 ± 49 nm and 349 ± 56 nm for AgNPs contents of 0, 4, 2, 1.3, and 0.7 wt.%, respectively | The polymer solution was put into a syringe with a Luer lock and 22 G metal blunt needle. A high-voltage DC power supply was used to electro-spin it on an aluminum foil covered rotating mandrel at 23 kV with a 1 mL/h feed rate and a needle tip-to-collector distance was 15 cm. | [62] |
| Ultrafine Cellulose Acetate Fibers | Ag | S. aureus, E. coli, K. pneumoniae, and P. aeruginosa | The average diameters of the cellulose acetate fibers electrospun with 0.05 and 0.5 wt.% AgNO3 were 3.3 and 6.9 nm, respectively | Distance of 10 cm from the needle tip to the ground electrode and a flow rate of 3 mL/h, CA solutions electrospun at a voltage of 17 kV. | [66] |
| Electrospun PVA Nanofibrous Membranes Impregnated Cellulosic Fibers | Ag | S. aureus | 169 nm | the prepared electrospinning solution was placed in a 10 mL needle tube, and 17 G needles were installed. The spinning distance was adjusted to 15 cm. Voltage was supplied between the needle tip (+14.0 kV) and the roller collector (−3.50 kV) covered with aluminum foil. The collector speed of 80 r/min was chosen. The temperature and relative humidity were kept at (25 ± 5) °C and 30% ± 5%, respectively. | [67] |
5.2. Electrospun Wound Dressings Loaded with Bioactive Molecules
5.2.1. Growth Factors and Cytokines
5.2.2. Vitamins
5.2.3. Anti-Inflammatory Agents
6. Wound Healing Using Bio-Based Electrospun Fibers
6.1. Biomaterials for Wound Healing: Cellulose Electrospun Nanofibers
6.2. Chitosan Electrospun Nanofibers for Wound Healing
6.3. Effectiveness of Electrospun PLA Nanofibrous Scaffolds in Wound Healing
6.4. Utilization of Electrospun PHA Nanofibrous Scaffolds in Wound Healing
6.5. Evaluation of Electrospun PCL Nanofibrous Scaffolds in Wound Healing
6.6. Utilization of PES Electrospun Fibers in Wound Healing
6.7. Wound Healing Using PS Electrospun Fibers
6.8. Application of PAA Electrospun Fibers in Wound Healing
6.9. Application of Thermoresponsive Electrospun Fibers in Wound Healing
7. Current Commercial Electrospun Wound Dressing
8. Chronic Diabetic Wound Healing Based on Electrospun Nanofibers
9. Conclusion and Future Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Khan, N. Applications of electrospun nanofibers in the biomedical field. SURG J. 2012, 5, 63–73. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Li, J.; Ren, G.; Lv, L. Wound healing and inflammation characteristics of the submicrometric mats prepared from electrospinning. J. Bioact. Compat. Polym. 2019, 34, 83–96. [Google Scholar] [CrossRef]
- Zahedi, E.; Esmaeili, A.; Eslahi, N.; Shokrgozar, M.A.; Simchi, A. Fabrication and characterization of core-shell electrospun fibrous mats containing medicinal herbs for wound healing and skin tissue engineering. Mar. Drugs 2019, 17, 27. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Odhav, A.; Kollipara, R.; Fike, J.; Stanford, C.; Hall, J.C. Acute cutaneous necrosis: A guide to early diagnosis and treatment. J. Cutan. Med. Surg. 2017, 21, 425–437. [Google Scholar] [CrossRef]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef]
- Guo, S.a.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Ward, J.; Holden, J.; Grob, M.; Soldin, M. Management of wounds in the community: Five principles. Br. J. Community Nurs. 2019, 24 (Suppl. S6), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health. J. Tissue Viability 2021, 30, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustoe, T. Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and inflammation in chronic wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, S.M.; Percival, S.L. Proteases and delayed wound healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Humphrey, P.R.; Maureen, M.D.; Ritter, J.M. Rang & Dale’s Pharmacology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hoffman, M. Remodeling the blood coagulation cascade. J. Thromb. Thrombolysis 2003, 16, 17–20. [Google Scholar] [CrossRef]
- Rivera-Caravaca, J.M.; Camelo-Castillo, A.; Ramírez-Macías, I.; Gil-Pérez, P.; López-García, C.; Esteve-Pastor, M.A.; Orenes-Piñero, E.; Tello-Montoliu, A.; Marín, F. Antithrombotic therapy in patients with peripheral artery disease: A focused review on oral anticoagulation. Int. J. Mol. Sci. 2021, 22, 7113. [Google Scholar] [CrossRef]
- Schultz, G.; Chin, G.; Moldawer, L.; Diegelmann, R. Ch23 Mechanisms of Vascular Disease; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of electrospun drug-eluting nanofibers in wound healing: Current and future perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous scarring: Basic science, current treatments, and future directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef] [Green Version]
- George Broughton, I.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef] [Green Version]
- Ben Amar, M.; Wu, M. Re-epithelialization: Advancing epithelium frontier during wound healing. J. R. Soc. Interface 2014, 11, 20131038. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Gonzalez, A.C.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning piezoelectric fibers for biocompatible devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef] [PubMed]
- Guenday, C.; Anand, S.; Gencer, H.B.; Munafo, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Tuereli, N.G. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Laudenslager, M.J.; Sigmund, W.M. Electrospinning. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 769–775. [Google Scholar]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Baumgarten, P.K. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Theron, S.; Zussman, E.; Yarin, A. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Matabola, K.; Moutloali, R. The influence of electrospinning parameters on the morphology and diameter of poly (vinyledene fluoride) nanofibers-effect of sodium chloride. J. Mater. Sci. 2013, 48, 5475–5482. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef] [Green Version]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Kanani, A.G.; Bahrami, S.H. Effect of changing solvents on poly (ε-caprolactone) nanofibrous webs morphology. J. Nanomater. 2011, 2011, 724153. [Google Scholar]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Huang, Z.; Ramakrishna, S.; Lim, C. Fabrication of porous electrospun nanofibres. Nanotechnology 2006, 17, 901. [Google Scholar] [CrossRef]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Dodero, A.; Brunengo, E.; Alloisio, M.; Sionkowska, A.; Vicini, S.; Castellano, M. Chitosan-based electrospun membranes: Effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020, 235, 115976. [Google Scholar] [CrossRef]
- Zeng, J.; Haoqing, H.; Schaper, A.; Wendorff, J.H.; Greiner, A. Poly-L-lactide nanofibers by electrospinning–Influence of solution viscosity and electrical conductivity on fiber diameter and fiber morphology. e-Polymers 2003, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of linear homopolymers of poly (methyl methacrylate): Exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef]
- Zuo, W.; Zhu, M.; Yang, W.; Yu, H.; Chen, Y.; Zhang, Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym. Eng. Sci. 2005, 45, 704–709. [Google Scholar] [CrossRef]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmanan, V.-K.; Biswas, R.; Nair, S.V.; Jayakumar, R. Synthesis and biological evaluation of chitin hydrogel/nano ZnO composite bandage as antibacterial wound dressing. J. Biomed. Nanotechnol. 2012, 8, 891–900. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.M.; El-Aassar, M.; Al-Deyab, S.S. Antimicrobial activity of carboxymethyl chitosan/polyethylene oxide nanofibers embedded silver nanoparticles. Carbohydr. Polym. 2013, 92, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Dastidar, D.G.; Ghosh, D. Silver nanoparticle decorated chitosan scaffold for wound healing and tissue regeneration. Macromolecules 2018, 105 Pt 1, 1241–1249. [Google Scholar]
- Ganesh, M.; Aziz, A.S.; Ubaidulla, U.; Hemalatha, P.; Saravanakumar, A.; Ravikumar, R.; Peng, M.M.; Choi, E.Y.; Jang, H.T. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: Synthesis and evaluation on synergism in wound healing. J. Ind. Eng. Chem. 2016, 39, 127–135. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Ko, W.-K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.-C. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef]
- Bharathi, B.S.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar] [CrossRef]
- Eghbalifam, N.; Shojaosadati, S.A.; Hashemi-Najafabadi, S.; Khorasani, A.C. Synthesis and characterization of antimicrobial wound dressing material based on silver nanoparticles loaded gum Arabic nanofibers. Int. J. Biol. Macromol. 2020, 155, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Bai, T.; Cheng, W.; Han, G.; Ni, X.; Shi, Q.S. Electrospun PVA Nanofibrous Membranes Reinforced with Silver Nanoparticles Impregnated Cellulosic Fibers: Morphology and Antibacterial Property. Chem. Res. Chin. Univ. 2021, 37, 505–511. [Google Scholar] [CrossRef]
- Lee, E.J.; Huh, B.K.; Kim, S.N.; Lee, J.Y.; Park, C.G.; Mikos, A.G.; Choy, Y.B. Application of materials as medical devices with localized drug delivery capabilities for enhanced wound repair. Prog. Mater. Sci. 2017, 89, 392–410. [Google Scholar] [CrossRef]
- Schneider, A.; Wang, X.; Kaplan, D.; Garlick, J.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Değer, S. Preparation and Characterization of Herbal Extract Loaded Bilayer Sponges for Wound Dressing Applications. Ph.D. Thesis, Izmir Institute of Technology, Urla, Turkey, 2019. [Google Scholar]
- Norouzi, M.; Shabani, I.; Ahvaz, H.H.; Soleimani, M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 2225–2235. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Controlled release of multiple epidermal induction factors through core–shell nanofibers for skin regeneration. Eur. J. Pharm. Biopharm. 2013, 85, 689–698. [Google Scholar] [CrossRef]
- Kumar, S.A. Wound healing: Current understanding and future prospect. Int. J. Drug Discov. ISSN 2017, 0975–4423. [Google Scholar]
- Stechmiller, J.K. Understanding the role of nutrition and wound healing. Nutr. Clin. Pract. 2010, 25, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Fan, L.; He, C.; Zhang, K.; Mo, X.; Wang, H. Vitamin E-loaded silk fibroin nanofibrous mats fabricated by green process for skin care application. Int. J. Biol. Macromol. 2013, 56, 49–56. [Google Scholar] [CrossRef]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly (vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surf. B Biointerfaces 2018, 166, 9–16. [Google Scholar] [CrossRef]
- Fan, L.; Wang, H.; Zhang, K.; Cai, Z.; He, C.; Sheng, X.; Mo, X. Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv. 2012, 2, 4110–4119. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Yun, C.-W.; Park, W.-K.; Kong, J.-Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.-K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Deldar, Y.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Montazer Saheb, S.; Rahmati-Yamchi, M.; Zarghami, N. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 706–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohiti-Asli, M.; Saha, S.; Murphy, S.; Gracz, H.; Pourdeyhimi, B.; Atala, A.; Loboa, E. Ibuprofen loaded PLA nanofibrous scaffolds increase proliferation of human skin cells in vitro and promote healing of full thickness incision wounds in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial peptide-based electrospun fibers for wound healing applications. Macromol. Biosci. 2019, 19, 1800488. [Google Scholar] [CrossRef]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef]
- Zhao, J.; Han, F.; Zhang, W.; Yang, Y.; You, D.; Li, L. Toward improved wound dressings: Effects of polydopamine-decorated poly (lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019, 9, 33038–33051. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C 2019, 105, 110083. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Wang, D.; Yao, J. Electrospun poly (3-hydroxybutyrate-co-3-hydroxy-valerate)/cellulose reinforced nanofibrous membranes with ZnO nanocrystals for antibacterial wound dressings. Cellulose 2017, 24, 2925–2938. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly (L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Kessler, M.R. Handbook of Composites from Renewable Materials, Biodegradable Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 5. [Google Scholar]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown Jr, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Zhang, X.; Xiao, Q.; Zhang, W.; Lu, C. Tissue engineering scaffolds electrospun from cotton cellulose. Carbohydr. Polym. 2015, 115, 485–493. [Google Scholar] [CrossRef]
- Rao, S.S.; Jeyapal, S.G.; Rajiv, S. Biodegradable electrospun nanocomposite fibers based on Poly (2-hydroxy ethyl methacrylate) and bamboo cellulose. Compos. Part B Eng. 2014, 60, 43–48. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Min, B.-M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]
- Zarghami, A.; Irani, M.; Mostafazadeh, A.; Golpour, M.; Heidarinasab, A.; Haririan, I. Fabrication of PEO/chitosan/PCL/olive oil nanofibrous scaffolds for wound dressing applications. Fibers Polym. 2015, 16, 1201–1212. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly (glycerol sebacate) and poly-l-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Alves, P.E.; Soares, B.G.; Lins, L.C.; Livi, S.; Santos, E.P. Controlled delivery of dexamethasone and betamethasone from PLA electrospun fibers: A comparative study. Eur. Polym. J. 2019, 117, 1–9. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Yang, C.; Yan, Z.; Lian, Y.; Wang, J.; Zhang, K. Graphene oxide coated shell-core structured chitosan/PLLA nanofibrous scaffolds for wound dressing. J. Biomater. Sci. Polym. Ed. 2020, 31, 622–641. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 8573–8588. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.; Sandeep, K.; Singh, M.; Singh, G.P.; Lee, J.-K.; Bhatia, S.K.; Kalia, V.C. Biotechnological application of polyhydroxyalkanoates and their composites as anti-microbials agents. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 207–225. [Google Scholar]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wang, Y.; Chen, G.-Q. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- Yang, X.-D.; Zou, X.-H.; Dai, Z.-W.; Luo, R.-C.; Wei, C.-J.; Chen, G.-Q. Effects of oligo (3-hydroxyalkanoates) on the viability and insulin secretion of murine beta cells. J. Biomater. Sci. Polym. Ed. 2009, 20, 1729–1746. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, J.; Xing, Z.; Shim, K.J.; Han, I.; Kim, J.C.; Kang, I.K.; Shen, J. Novel wound dressing based on nanofibrous PHBV–keratin mats. J. Tissue Eng. Regen. Med. 2015, 9, 1027–1035. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B. Electrosprayed chitin nanofibril/electrospun polyhydroxyalkanoate fiber mesh as functional nonwoven for skin application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef] [PubMed]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. Part A 2015, 103, 2482–2498. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Baptista-Silva, S.; Sousa, A.; Oliveira, A.; Bártolo, P.; Granja, P. Biomechanical performance of hybrid electrospun structures for skin regeneration. Mater. Sci. Eng. C 2018, 93, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei-Parsa, M.J.; Ghanbari, H.; Alipoor, B.; Tavakoli, A.; Najafabadi, M.R.H.; Faridi-Majidi, R. Nanofiber-acellular dermal matrix as a bilayer scaffold containing mesenchymal stem cell for healing of full-thickness skin wounds. Cell Tissue Res. 2019, 375, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar]
- Tığlı, R.S.; Kazaroğlu, N.M.; Mavış, B.; Gümüşderelioğlu, M. Cellular behavior on epidermal growth factor (EGF)-immobilized PCL/gelatin nanofibrous scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 207–223. [Google Scholar] [CrossRef]
- Kasafírek, E.; Rybák, M.; Krejčí, I.; Šturc, A.; Křepela, E.; Šedo, A. Two-step generation of spirocyclic dipeptides from linear peptide ethyl ester precursors. Life Sci. 1992, 50, 187–193. [Google Scholar] [CrossRef]
- Pokorna, A.; Bobal, P.; Oravec, M.; Rarova, L.; Bobalova, J.; Jampilek, J. Investigation of permeation of theophylline through skin using selected piperazine-2, 5-diones. Molecules 2019, 24, 566. [Google Scholar] [CrossRef] [Green Version]
- Kocic, H.; Arsic, I.; Stankovic, M.; Tiodorovic, D.; Ciric, V.; Kocic, G. Proliferative, anti-apoptotic and immune-enhancing effects of L-arginine in culture of skin fibroblasts. J. Biol. Regul. Homeost. Agents 2017, 31, 667–672. [Google Scholar] [PubMed]
- Kanji, S.; Das, M.; Aggarwal, R.; Lu, J.; Joseph, M.; Basu, S.; Pompili, V.J.; Das, H. Nanofiber-expanded human umbilical cord blood-derived CD34+ cell therapy accelerates murine cutaneous wound closure by attenuating pro-inflammatory factors and secreting IL-10. Stem Cell Res. 2014, 12, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly (ε-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Mai, S.; Norton, D.; Haycock, J.W.; Ryan, A.J.; Macneil, S. Self-organization of skin cells in three-dimensional electrospun polystyrene scaffolds. Tissue Eng. 2005, 11, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.; Dumych, T.; Paryzhak, S. Reduced graphene-oxide-embedded polymeric nanofiber mats: An “on-demand” photothermally triggered antibiotic release platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef]
- Johnson, C.D.; D’Amato, A.R.; Puhl, D.L.; Wich, D.M.; Vesperman, A.; Gilbert, R.J. Electrospun fiber surface nanotopography influences astrocyte-mediated neurite outgrowth. Biomed. Mater. 2018, 13, 054101. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.-S.; Liu, S.-J. Core-shell insulin-loaded nanofibrous scaffolds for repairing diabetic wounds. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef]
- Teng, S.-H.; Wang, P.; Kim, H.-E. Blend fibers of chitosan–agarose by electrospinning. Mater. Lett. 2009, 63, 2510–2512. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef]
- Parai, D.; Dey, P.; Mukherjee, S.K. Biofilm: A Challenge to Overcome in Wound Healing. In Wound Healing Research: Current Trends and Future Directions; Springer: Singapore, 2021; pp. 661–677. [Google Scholar]
- Hosseini, M.; Shafiee, A. Engineering bioactive scaffolds for skin regeneration. Small 2021, 17, 2101384. [Google Scholar] [CrossRef]
- Silva, S.Y.; Rueda, L.C.; Márquez, G.A.; López, M.; Smith, D.J.; Calderón, C.A.; Castillo, J.C.; Matute, J.; Rueda-Clausen, C.F.; Orduz, A. Double blind, randomized, placebo controlled clinical trial for the treatment of diabetic foot ulcers, using a nitric oxide releasing patch: PATHON. Trials 2007, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Floyd, C.T.; Rothwell, S.W.; Risdahl, J.; Martin, R.; Olson, C.; Rose, N. Salmon thrombin-fibrinogen dressing allows greater survival and preserves distal blood flow compared to standard kaolin gauze in coagulopathic Swine with a standardized lethal femoral artery injury. J. Spec. Oper. Med. A Peer Rev. J. SOF Med. Prof. 2012, 12, 16–26. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.-E.A. Cerium oxide nanoparticle incorporated electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) membranes for diabetic wound healing applications. ACS Biomater. Sci. Eng. 2019, 6, 58–70. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chang, S.-H.; Chen, W.-J.; Hung, K.-C.; Lin, Y.-H.; Liu, S.-J.; Hsieh, M.-J.; Pang, J.-H.S.; Juang, J.-H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J. Colloid Interface Sci. 2015, 439, 88–97. [Google Scholar] [CrossRef]
- Yu, J.-W.; Deng, Y.-P.; Han, X.; Ren, G.-F.; Cai, J.; Jiang, G.-J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.R.; Kargozar, S.; Bahrami, S.; Rabbani, S. An excellent nanofibrous matrix based on gum tragacanth-poly (Ɛ-caprolactone)-poly (vinyl alcohol) for application in diabetic wound healing. Polym. Degrad. Stab. 2020, 174, 109105. [Google Scholar] [CrossRef]





| Growth Factor | Polymer | Technique Used | Fiber Diameter | Release Profile | Main Findings | Reference |
|---|---|---|---|---|---|---|
| Epidermal growth factor (EGF) | Gelatin and a combination of lactic acid and glycolic acid (PLGA) | Emulsion electrospinning |
| The EGF release profile shows a rapid release on day 1, and continuous release throughout the course of nine days (total EGF release was 4.2 ± 0.2 ng/mL). | The MTT experiment revealed that fi fibroblast proliferation was greater on PLGA/EGF/gelatin membranes. | [73] |
| EGF, insulin, hydrocortisone, and retinoic acid | Gelatin/ PLLCL | Blend electrospinning: gelatin/PLLCL (b) Co-axial electrospinning: gelatin/PLLCL (cs) |
|
| The ASCs (adipose-derived stem cells) multiplied to a greater extent (43.6%) when in a combination of gelatin/PLLCL/EIF (cs) than when in gelatin/PLLCL/EIF alone. (b) The proportion of differentiated epidermal cells was 62.2% and 43.0% for gelatin/PLLCL/EIF (cs) and gelatin/PLLCL/EIF (b) membranes, respectively. | [74] |
| Vitamins | Polymer | Technique used | Fiber diameter | Encapsulation efficiency | Main conclusions | Reference |
| Vitamin E | SF | Blend electrospinning |
|
| In the initial 30 min, there was a rapid release of the drug observed from Vit E, which was then followed by a gradual release over the next 72 h. Moreover, supplementing L929 cells with Vitamin E allowed them to adhere better and grow faster. | [78] |
| Vitamin E | SF/PVA/AV | Vit E loaded into starch nanoparticles and blend with electrospinning solution |
| Vitamin E into starch nanoparticles was 91.63%. | Vitamin E released quickly at first, then slowly over the course of 144 h; The higher Vit E level led to enhanced antioxidant activity. In contact with electrospun membranes, the fibroblasts cells continued to be functional, adhering and dividing. | [79] |
| Vit C | Silk Fibroin | Blend electrospinning |
| NA | A burst peak was visible in the Vit C release profile from SF nanofibers during the first 20 min. Vitamin C had a positive effect on the viability of fibroblasts, as well as boosting the mRNA levels of key genes such as Col1a1, Gpx1, and Cat. | [80] |
| Anti-inflammatory molecule | Polymer | Technique used | Fiber diameter | Release profile | Main findings | Reference |
| Curcumin | Polycaprolactone | Blend electrospinning |
|
| The antioxidant qualities of fibers loaded with curcumin were further demonstrated by the Oxygen Radical Absorbance Capacity (ORAC) assay. The membranes had a cytoprotective effect on human fibroblast cells and were biocompatible. In vivo tests conducted in a living organism demonstrated that PCL/curcumin nanofibers had the capacity to accelerate the recovery of injuries in a diabetic mouse model. | [83] |
| Chrysin (Chr) | PCL/PEG | Blend electrospinning |
|
| It was demonstrated that the nanofibers, which had antioxidant properties and were biocompatible, had cytoprotective effects on human fibroblast cells when they were loaded with Chrysin. The reduced levels of IL-6, IL-1β, tumor necrosis factor-α, and NO in macrophages showed the anti-inflammatory capabilities of Chrysin-filled nanofibrous mats. | [85] |
| Ibuprofen | PLA | Blend electrospinning |
| 0.25 milligrams of ibuprofen was released from poly lactic acid/ibuprofen (30%) nanofibers after 336 h. | The nanofibers with 20 wt.% IBP had the highest cell viability and proliferation. The addition of IBP to PLA nanofibers encouraged the growth and viability of both HEK and HDF. | [87] |
| Electrospun Mesh | Incorporated Therapeutics | Function and Wound Type | References |
|---|---|---|---|
| Chitosan/Poly (l-lactide) | Graphene oxide | Antimicrobial action in infected chronic injuries. | [88] |
| Chitosan/keratin/polycaprolactone | Aloe vera extract | Burn and acute wounds can be aided by properties that are anti-inflammatory, antibacterial, antiviral, and antioxidant. | [7] |
| Polyhydroxyalkanoates | Dodecyl trimethylammonium chloride biocide | The antioxidant, anti-inflammatory, and anti-infective qualities of certain substances can provide a boost to cell reinforcement and angiogenic properties for diabetic injuries, resulting in antimicrobial effects for chronic wounds. | [89] |
| Polydopamine or polylactic glycolic acid | Fibroblast growth factor and ponericin G1 are both present | The skin tissue regeneration process has antibacterial and cell growth-promoting properties. | [90] |
| Chitosan/Polyvinyl alcohol | Nanobioglass | For chronic injuries, biocompatibility, antimicrobial action, and recovery advancement. | [91] |
| PLGA | Ciprofloxacin | Antibacterial and skin tissue regenerative effects that encourage cell growth | [90] |
| PLA | Doxycycline | Antibacterial activity, chronic wounds. | [92] |
| PHBV/cellulose | Zinc Oxide nanocrystals | Antibacterial activity in wounds that are both acute and infected. | [93] |
| PLLA | Curcumin | Antioxidant, anti-inflammatory effects. | [94] |
| CA/polyester urethane | Polyhexamethylene biguanide | Antimicrobial activity. | [95] |
| Electrospun Scaffolds | Main Findings | References |
|---|---|---|
| Electrospun fibers fabricated from a combination of Paclitaxel and (2-hydroxyethyl methacrylate)/bamboo cellulose were created. | This structure can be used to combat skin cancer and increase wound recovery. | [101] |
| Cellulose that has been carboxymethylated and CA electrospun fibers that are shaped like ribbons and contain silver nanoparticles. | The addition of silver nanoparticles improved the antibacterial and healing abilities of CMC fibers. | [102] |
| Nanofibrous networks fabricated of chitin and chitosan can be created by utilizing 1,1,1,3,3,3-hexafluoro-2-propanol as a spinning solvent. | Chitosan stimulates macrophages to aid in wound repair, causing polymorphonuclear neutrophils to move to the wound site at the initial time of healing. | [136] |
| PEO/chitosan/PCL/oliveoil (Composite fibers). | Composite fibers exhibited antibacterial action S. aureus and E. coli, a 0.6-fold decrease in edema, facilitated cell growth, and proliferation. | [106] |
| A fibrous mesh composed of both poly (glycerol sebacate) and poly L-lactic acid was created via coaxial electrospinning. | Exhibited cell proliferation, with a lower inflammatory response, re-establish pores and skin wound tissues. | [107] |
| Collagen-coated PHB/gelatin/ostholamide (OSA) Electrospun Nanofiber mesh | Nanofiber mesh shows excellent mechanical stability, reliable enzymatic breakdown, and effective antibacterial development towards Pseudomonas aeruginosa and Staphylococcus aureus. | [119] |
| Electrosprayed fiber networks composed of poly(3-hydroxybutyrate)/poly(3-hydroxyoctanoate-co-3-hydroxydecanoate) with a complex of chitin-lignin/glycyrrhizin acid. | CLA complexes useful bio-based mixtures for functionalizing pores and skin contact substrates in an in vitro skin model and helpful in wound healing. | [117] |
| PCL-gelatin electrospun nanoscaffolds incorporated with quercetin and ciprofoxacin hydrochloride (CH). | The full thickness wound was healed in 16 days. | [137] |
| Electrospun nanofibers containing Polyacrylic acid, and a synthetic biodegradable elastomer called poly(1,8-octanediol-co-citric acid). | Excellent antibacterial activity and delivery of physiologically relevant growth factor concentrations topically. | [132] |
| Electrospun mesh of polystyrene incorporated with p chamomile extract and poly(caprolactone). | Excellent wound-healing properties. | [129] |
| Product | Polymer | Device | References |
|---|---|---|---|
| Pathon | Polyurethane | Composite mesh, NO drug deliver | [140] |
| SurgiCLOT® | Dextran | Fibrin Sealant Patch | [141] |
| SpinCare™ | Various electrospinnable polymers | Portable electrospinning wound dressing device | [142] |
| Tecophilic™ | Polyurethane-PEG | Photosensitizing-loaded mesh | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Srivastava, S.; Kumar, S.; Sharma, P.K.; Hassani, R.; Dailah, H.G.; Khalid, A.; Mohan, S. Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review. Pharmaceuticals 2023, 16, 325. https://doi.org/10.3390/ph16020325
Sharma D, Srivastava S, Kumar S, Sharma PK, Hassani R, Dailah HG, Khalid A, Mohan S. Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review. Pharmaceuticals. 2023; 16(2):325. https://doi.org/10.3390/ph16020325
Chicago/Turabian StyleSharma, Deepika, Shriyansh Srivastava, Sachin Kumar, Pramod Kumar Sharma, Rym Hassani, Hamad Ghaleb Dailah, Asaad Khalid, and Syam Mohan. 2023. "Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review" Pharmaceuticals 16, no. 2: 325. https://doi.org/10.3390/ph16020325
APA StyleSharma, D., Srivastava, S., Kumar, S., Sharma, P. K., Hassani, R., Dailah, H. G., Khalid, A., & Mohan, S. (2023). Biodegradable Electrospun Scaffolds as an Emerging Tool for Skin Wound Regeneration: A Comprehensive Review. Pharmaceuticals, 16(2), 325. https://doi.org/10.3390/ph16020325