Amantadine in Treatment of Dysthymia—The Pilot Case Series Study
Abstract
:1. Introduction
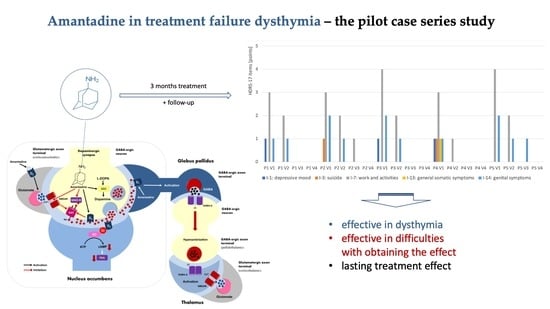
2. Results
Individual Case Reports Are Summarized Below
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, E.; Klein, D.N.; Elsaesser, M.; Furukawa, T.A.; Domschke, K. Review of dysthymia and persistent depressive disorder: History, correlates, and clinical implications. Lancet Psychiatry 2020, 7, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.N.; Shankman, S.A.; Rose, S. Ten-year prospective follow-up study of the naturalistic course of dysthymic disorder and double depression. Am. J. Psychiatry 2006, 163, 872–880. [Google Scholar] [CrossRef]
- Carta, M.G.; Paribello, P.; Nardi, A.E.; Preti, A. Current pharmacotherapeutic approaches for dysthymic disorder and persistent depressive disorder. Expert Opin. Pharmacother. 2019, 20, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; von Wolff, A.; Mohr, H.; Härter, M.; Nestoriuc, Y.; Hölzel, L.; Kriston, L. Comparative Safety of Pharmacologic Treatments for Persistent Depressive Disorder: A Systematic Review and Network Meta-Analysis. PLoS ONE 2016, 11, e0153380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale, S.; Espejel, M.A.; Dominguez, J.C. Amantadine in depression. Lancet 1971, 2, 437. [Google Scholar] [CrossRef]
- Huber, T.J.; Dietrich, D.E.; Emrich, H.M. Possible use of amantadine in depression. Pharmacopsychiatry 1999, 32, 47–55. [Google Scholar] [CrossRef]
- Szmulewicz, A.G.; Angriman, F.; Samamé, C.; Ferraris, A.; Vigo, D.; Strejilevich, S.A. Dopaminergic agents in the treatment of bipolar depression: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2017, 135, 527–538. [Google Scholar] [CrossRef]
- Berk, M.; Dodd, S.; Kauer-Sant’anna, M.; Malhi, G.S.; Bourin, M.; Kapczinski, F.; Norman, T. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. Suppl. 2007, 434, 41–49. [Google Scholar] [CrossRef]
- Rogóz, Z.; Skuza, G.; Daniel, W.A.; Wójcikowski, J.; Dudek, D.; Wróbel, A. Amantadine as an additive treatment in patients suffering from drug-resistant unipolar depression. Pharmacol. Rep. 2007, 59, 778–784. [Google Scholar]
- Stryjer, R.; Strous, R.D.; Shaked, G.; Bar, F.; Feldman, B.; Kotler, M.; Polak, L.; Rosenzcwaig, S.; Weizman, A. Amantadine as augmentation therapy in the management of treatment-resistant depression. Int. Clin. Psychopharmacol. 2003, 18, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Krzystanek, M.; Pałasz, A. Possibility of a New Indication for Amantadine in the Treatment of Bipolar Depression-Case Series Study. Pharmaceuticals 2020, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.; Santaguida, P.; Keshavarz, H.; Jaworska, N.; Levine, M.; Beyene, J.; Raina, P. Systematic Review of Clinical Practice Guidelines for Failed Antidepressant Treatment Response in Major Depressive Disorder, Dysthymia, and Subthreshold Depression in Adults. Can. J. Psychiatry 2017, 62, 11–23. [Google Scholar] [CrossRef]
- Nikolaus, S.; Wittsack, H.J.; Beu, M.; Antke, C.; Hautzel, H.; Wickrath, F.; Müller-Lutz, A.; De Souza Silva, M.A.; Huston, J.P.; Antoch, G.; et al. Amantadine enhances nigrostriatal and mesolimbic dopamine function in the rat brain in relation to motor and exploratory activity. Pharmacol. Biochem. Behav. 2019, 179, 156–170. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamashita, H.; Zhang, Y.X.; Nakamura, S. Inhibitory effect of MK-801 on amantadine-induced dopamine release in the rat striatum. Brain Res. Bull. 1996, 41, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.M.; Miller, G.A. A dual-task analysis of resource allocation in dysthymia and anhedonia. J. Abnorm. Psychol. 1994, 103, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.; Visacri, M.B.; Aguiar, P.M. Use of ketamine and esketamine for depression: An overview of systematic reviews with meta-analyses. Eur. J. Clin. Pharmacol. 2022, 78, 311–338. [Google Scholar] [CrossRef]
- Wong, J.; Motulsky, A.; Eguale, T.; Buckeridge, D.L.; Abrahamowicz, M.; Tamblyn, R. Treatment Indications for Antidepressants Prescribed in Primary Care in Quebec, Canada, 2006–2015. JAMA 2016, 315, 2230–2232. [Google Scholar] [CrossRef] [Green Version]
- Skånland, S.S.; Cieślar-Pobuda, A. Off-label uses of drugs for depression. Eur. J. Pharmacol. 2019, 865, 172732. [Google Scholar] [CrossRef]
- Kosiorek, A.; Konieczny, D. Leczenie poza wskazaniami z ChPL [Off-label treatment in the SmPC]. Gaz. Lek. 2022, 7–8, 28. [Google Scholar]
- Gray, C.M.; Grimson, F.; Layton, D.; Pocock, S.; Kim, J. A framework for methodological choice and evidence assessment for studies using external comparators from real-world data. Drug Saf. 2020, 43, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Visit No. | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Mean [95% CI] | Statistical Difference |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 6 | 7 | 7 | 6 | 6.2 [5.6; 6.8] | t(4) = 13.42; p < 0.001; d = 1.0 |
| 2 | 3 | 3 | 3 | 1 | 3 | 2.6 [1.8; 3.0] | |
| 3 | 0 | 1 | 0 | 0 | 1 | 0.4 [0.0; 0.8] | |
| 4 | 0 | 0 | 0 | 0 | 1 | 0.2 [0.0; 0.6] |
| Visit | Patient 1 Male | Patient 2 Female | Patient 3 Female | Patient 4 Female | Patient 5 Male | Mean [95% CI] | Statistical Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | S | C | S | C | S | C | S | C | S | C | S | C | U = 7.00; Z = −1.20; p = 0.230; ΔGlass = 1.19 |
| 1 | 5.0 | 6.0 | 6.0 | 7.0 | 7.0 | 6.0 | 7.0 | 8.0 | 6.0 | 9.0 | 6.2 [5.6; 6.8] | 7.2 [5.6; 8.8] | U = 6.00; Z = −1.68; p = 0.093; ΔGlass = 0.89 |
| 2 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 4.0 | 1.0 | 3.0 | 3.0 | 4.0 | 2.6 [1.8; 3.0] | 3.4 [2.7; 4.1] | U = 5.00; Z = −1.96; p = 0.050; ΔGlass = 1.09 |
| 3 | 0.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 0.0 | 1.0 | 1.0 | 1.0 | 0.4 [0.0; 0.8] | 1.0 [1.0; 1.0] | U = 10.00; Z = −1.00; p = 0.317; ΔGlass = 0.45 |
| 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.2 [0.0; 0.6] | 0.0 [0.0; 0.0] | U = 7.00; Z = −1.20; p = 0.230; ΔGlass = 1.19 |
| Study Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient Number (M-Male, W-Female) | Visit | I-1 Depressive Mood | I-3 Suicide | I-7 Work and Activities | I-8 Retardation | I-10 Anxiety, Psychosis | I-13 General Somatic Symptoms | I-14 Genital Symptoms | Sum |
| P1 (M) | V1 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 5 |
| V2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P2 (W) | V1 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 6 |
| V2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P3 (W) | V1 | 1 | 0 | 4 | 0 | 0 | 0 | 2 | 7 |
| V2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P4 (W) | V1 | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 7 |
| V2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P5 (M) | V1 | 0 | 0 | 4 | 0 | 0 | 0 | 2 | 6 |
| V2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Control Group | |||||||||
| Gender | Visit nr | I-1: Depressive Mood | I-3: Suicide | I-7: Work and Activities | I-8 Retardation | 10 Anxiety, Psychic | I-13: General Somatic Symptoms | I-14: Genital Symptoms | Sum |
| P1 (M) | V1 | 1 | 0 | 3 | 2 | 0 | 0 | 0 | 6 |
| V2 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 3 | |
| V3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P2 (W) | V1 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 7 |
| V2 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P3 (W) | V1 | 1 | 0 | 3 | 1 | 0 | 0 | 1 | 6 |
| V2 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 4 | |
| V3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P4 (W) | V1 | 1 | 0 | 3 | 2 | 0 | 0 | 2 | 8 |
| V2 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | |
| V3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P5 (M) | V1 | 1 | 1 | 3 | 2 | 0 | 0 | 2 | 9 |
| V2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 4 | |
| V3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| V4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Patients | Gender | Drug | Daily Dose [mg] | Age [years] | Year of Treatment |
|---|---|---|---|---|---|
| study group case 1 | male | amantadine | 100 | 34 | 2020 |
| study group case 2 | female | amantadine | 100 | 38 | 2019 |
| study group case 3 | female | amantadine | 100 | 42 | 2020 |
| study group case 4 | female | amantadine | 100 | 38 | 2019 |
| study group case 5 | male | amantadine | 100 | 44 | 2021 |
| control group case 1 | male | sertraline | 100 | 35 | 2018 |
| control group case 2 | female | sertraline | 100 | 38 | 2019 |
| control group case 3 | female | sertraline | 100 | 41 | 2022 |
| control group case 4 | female | sertraline | 100 | 36 | 2021 |
| control group case 5 | male | sertraline | 100 | 43 | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystanek, M.; Martyniak, E.; Pałasz, A.; Skałacka, K.; Chwalba, A.; Wierzbiński, P. Amantadine in Treatment of Dysthymia—The Pilot Case Series Study. Pharmaceuticals 2023, 16, 897. https://doi.org/10.3390/ph16060897
Krzystanek M, Martyniak E, Pałasz A, Skałacka K, Chwalba A, Wierzbiński P. Amantadine in Treatment of Dysthymia—The Pilot Case Series Study. Pharmaceuticals. 2023; 16(6):897. https://doi.org/10.3390/ph16060897
Chicago/Turabian StyleKrzystanek, Marek, Ewa Martyniak, Artur Pałasz, Katarzyna Skałacka, Artur Chwalba, and Piotr Wierzbiński. 2023. "Amantadine in Treatment of Dysthymia—The Pilot Case Series Study" Pharmaceuticals 16, no. 6: 897. https://doi.org/10.3390/ph16060897
APA StyleKrzystanek, M., Martyniak, E., Pałasz, A., Skałacka, K., Chwalba, A., & Wierzbiński, P. (2023). Amantadine in Treatment of Dysthymia—The Pilot Case Series Study. Pharmaceuticals, 16(6), 897. https://doi.org/10.3390/ph16060897