Effect of Growth Hormone and Estrogen Replacement Therapy on Bone Mineral Density in Women with Turner Syndrome: A Meta-Analysis and Systematic Review
Abstract
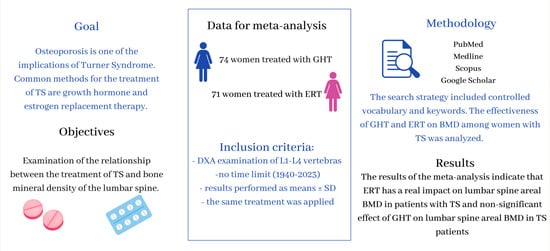
:1. Introduction
2. Results
3. Discussion
3.1. Correlation between Estrogen and BMD in Turner Syndrome
3.2. Polymorphisms of Estrogen Receptor and Effectiveness of ERT
3.3. Polymorphisms of Growth Hormone Receptor and Effectiveness of GHT
3.4. Correlation between GHT and BMD in TS
3.5. Genetic Influence
3.6. Advantages and Disadvantages of GHT
3.7. Advantages and Disadvantages of ERT
3.8. Side Effects of GHT and ERT Treatment
3.9. GHT
3.10. ERT
3.11. Vitamin D in Turner Syndrome
4. Materials and Methods
4.1. Database Search
4.2. Study Selection
- the same section of the skeleton was examined—lumbar spine values at L1–L4;
- using the same method of examination—dual-energy X-ray absorptiometry (DXA);
- the same treatment was applied: 1 group of articles: ERT and 2 group of articles: GHT;
- results performed as means ± SD.
4.3. Risk of Bias Assessment Method
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| DXA | dual-energy X-ray absorptiometry |
| GH | growth hormone |
| FSH | follicle-stimulating hormone |
| E2 | estradiol |
| EE | ethinyl estradiol |
| SD | standard deviation |
| BMAD | bone mineral apparent density |
| HR-pQCT | high-resolution peripheral quantitative computed tomography |
| TRAP | thyroid hormone receptor-associated protein |
| 1,25-(OH)2D3 | 1,25-dihydroxycholecalciferol |
| PTH | parathormone |
| GHR | growth hormone receptor |
| IGF-1 | insulin-like growth factor 1 |
| HRQOL | health-related quality of life |
| SCFE | Slipped capital femoral epiphysis |
| BIH | Benign Intracranial Hypertension |
| VTE | Venous Thromboembolism |
| VDR | Vitamin D receptor |
| PRP | platelet rich plasma |
References
- Ari, M.; Bakalov, V.K.; Hill, S.; Bondy, C.A. The effects of growth hormone treatment on bone mineral density and body composition in girls with turner syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Lacka, K. Turner’s syndrome-correlation between karyotype and phenotype. Endokrynol. Pol. 2005, 56, 986–993. [Google Scholar]
- Turner, H.H. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology 1938, 23, 566–574. [Google Scholar] [CrossRef]
- Andrade, N.S.; Tenório, J.R.; Gallottini, M. Supernumerary teeth in a patient with Turner syndrome: An unusual finding. Spec. Care Dentist. 2019, 39, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: Case report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Saenger, P. Turner Syndrome: An Update. Adv. Pediatr. 2022, 69, 177–202. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Lauridsen, A.L.; Brixen, K.; Mosekilde, L.; Heickendorff, L.; Christiansen, J.S. Marked disproportionality in bone size and mineral, and distinct abnormalities in bone markers and calcitropic hormones in adult turner syndrome: A cross-sectional study. J. Clin. Endocrinol. Metab. 2002, 87, 2798–2808. [Google Scholar] [CrossRef]
- Faienza, M.F.; Ventura, A.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Bone Fragility in Turner Syndrome: Mechanisms and Prevention Strategies. Front. Endocrinol. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Andersen, N.H.; Conway, G.S.; Dekkers, O.M.; Geffner, M.E.; Klein, K.O.; Lin, A.E.; Mauras, N.; Quigley, C.A.; Rubin, K.; et al. International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: Proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol. 2017, 177, G1–G70. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Bengtsson, B.A.; Isaksson, O.G.; Andreassen, T.T.; Slootweg, M.C. Growth hormone and bone. Endocr. Rev. 1998, 19, 55–79. [Google Scholar] [CrossRef]
- Li, L.; Qiu, X.; Lash, G.E.; Yuan, L.; Liang, Z.; Liu, L. Effect of Hormone Replacement Therapy on Bone Mineral Density and Body Composition in Chinese Adolescent and Young Adult Turner Syndrome Patients. Front. Endocrinol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Sowińska-Przepiera, E.; Andrysiak-Mamos, E.; Friebe, Z.; Kapczuk, K.; Pilarska, K. The effect of primary lack of estrogens and the influence of the age at the beginning of estrogen therapy on bone mineral density in patients with Turner’s syndrome. Endokrynol. Pol. 2005, 56, 145–153. [Google Scholar] [PubMed]
- Nakamura, T.; Tsuburai, T.; Tokinaga, A.; Nakajima, I.; Kitayama, R.; Imai, Y.; Nagata, T.; Yoshida, H.; Hirahara, F.; Sakakibara, H. Efficacy of estrogen replacement therapy (ERT) on uterine growth and acquisition of bone mass in patients with Turner syndrome. Endocr. J. 2015, 62, 965–970. [Google Scholar] [CrossRef]
- Bakalov, V.K.; Van, P.L.; Baron, J.; Reynolds, J.C.; Bondy, C.A. Growth hormone therapy and bone mineral density in Turner syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 4886–4889. [Google Scholar] [CrossRef]
- Nour, M.A.; Burt, L.A.; Perry, R.J.; Stephure, D.K.; Hanley, D.A.; Boyd, S.K. Impact of Growth Hormone on Adult Bone Quality in Turner Syndrome: A HR-pQCT Study. Calcif. Tissue Int. 2016, 98, 49–59. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 0108021–01080215. [Google Scholar] [CrossRef]
- Marks, S.C., Jr.; Popoff, S.N. Bone cell biology: The regulation of development, structure, and function in the skeleton. Am. J. Anat. 1988, 183, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Schmid, C.; Froesch, E.R. Enhanced osteoblast proliferation and collagen gene expression by estradiol. Proc. Natl. Acad. Sci. USA 1988, 85, 2307–2310. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005, 105, 2631–2639. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996, 45, 371–386. [Google Scholar]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomiyama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef]
- Kremer, M.; Judd, J.; Rifkin, B.; Auszmann, J.; Oursler, M.J. Estrogen modulation of osteoclast lysosomal enzyme secretion. J. Cell Biochem. 1995, 57, 271–279. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Kanis, J.A.; Adachi, J.D.; Cooper, C.; Clark, P.; Cummings, S.R.; Diaz-Curiel, M.; Harvey, N.; Hiligsmann, M.; Papaioannou, A.; Pierroz, D.D.; et al. Epidemiology and Quality of Life Working Group of IOF (2013). Standardising the descriptive epidemiology of osteoporosis: Recommendations from the Epidemiology and Quality of Life Working Group of IOF. Osteoporos Int. 2013, 24, 2763–2764. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately loaded dental implants bioactivated with platelet-rich plasma (PRP) placed in maxillary and mandibular region. Clin. Ter. 2015, 166, e146–e152. [Google Scholar] [CrossRef] [PubMed]
- Trovó de Marqui, A.B. Síndrome de Turner e polimorfismo genético: Uma revisão sistemática [Turner syndrome and genetic polymorphism: A systematic review]. Rev. Paul. Pediatr. 2015, 33, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Wong, P.; Strauss, B.J.; Jones, G.; Ebeling, P.R.; Milat, F.; Vincent, A. Delay in estrogen commencement is associated with lower bone mineral density in Turner syndrome. Climacteric 2017, 20, 436–441. [Google Scholar] [CrossRef]
- Sowińska-Przepiera, E.; Andrysiak-Mamos, E.; Chełstowski, K.; Adler, G.; Friebe, Z.; Syrenicz, A. Association between ER-α polymorphisms and bone mineral density in patients with Turner syndrome subjected to estroprogestagen treatment—A pilot study. J. Bone Miner. Metab. 2011, 29, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Lindberg, A. Predicting growth in response to growth hormone treatment. Growth Horm. IGF Res. 2009, 19, 1–11. [Google Scholar] [CrossRef]
- Bougnères, P.; Goffin, V. The growth hormone receptor in growth. Endocrinol. Metab. Clin. N. Am. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Sobrier, M.L.; Duquesnoy, P.; Duriez, B.; Amselem, S.; Goossens, M. Expression and binding properties of two isoforms of the human growth hormone receptor. FEBS Lett. 1993, 319, 16–20. [Google Scholar] [CrossRef]
- Dos Santos, C.; Essioux, L.; Teinturier, C.; Tauber, M.; Goffin, V.; Bougnères, P. A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat. Genet. 2004, 36, 720–724. [Google Scholar] [CrossRef]
- Wassenaar, M.J.; Dekkers, O.M.; Pereira, A.M.; Wit, J.M.; Smit, J.W.; Biermasz, N.R.; Romijn, J.A. Impact of the exon 3-deleted growth hormone (GH) receptor polymorphism on baseline height and the growth response to recombinant human GH therapy in GH-deficient (GHD) and non-GHD children with short stature: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2009, 94, 3721–3730. [Google Scholar] [CrossRef]
- Renehan, A.G.; Solomon, M.; Zwahlen, M.; Morjaria, R.; Whatmore, A.; Audí, L.; Binder, G.; Blum, W.; Bougnères, P.; Santos, C.D.; et al. Growth hormone receptor polymorphism and growth hormone therapy response in children: A Bayesian meta-analysis. Am. J. Epidemiol. 2012, 175, 867–877. [Google Scholar] [CrossRef] [PubMed]
- García-Rojas, M.D.; Palma-Cordero, G.; Martínez-Ramírez, C.O.; Ponce de León-Suárez, V.; Valdés-Flores, M.; Castro-Hernández, C.; Rubio-Lightbourn, J.; Hernández-Zamora, E.; Reyes-Maldonado, E.; Velázquez-Cruz, R.; et al. Association of Polymorphisms in Estrogen Receptor Genes (ESR1 and ESR2) with Osteoporosis and Fracture-Involvement of Comorbidities and Epistasis. DNA Cell Biol. 2022, 41, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A.; Klibanski, A. Effects of Growth Hormone on Bone. Prog. Mol. Biol. Transl. Sci. 2016, 138, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Sakata, T.; Leary, C.; Elalieh, H.; Ginzinger, D.; Rosen, C.J.; Beamer, W.; Majumdar, S.; Halloran, B.P. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J. Bone Miner. Res. 2002, 17, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Olney, R.C. Regulation of bone mass by growth hormone. Med. Pediatr. Oncol. 2003, 41, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sas, T.C.; de Muinck Keizer-Schrama, S.M.; Stijnen, T.; van Teunenbroek, A.; van Leeuwen, W.J.; Asarfi, A.; van Rijn, R.R.; Drop, S.L. Dutch Advisory Group on Growth Hormone (2001). Bone mineral density assessed by phalangeal radiographic absorptiometry before and during long-term growth hormone treatment in girls with Turner’s syndrome participating in a randomized dose-response study. Pediatr. Res. 2001, 50, 417–422. [Google Scholar] [CrossRef]
- Peralta López, M.; Miras, M.; Silvano, L.; Pérez, A.; Muñoz, L.; Centeno, V.; Sobrero, G.; Ulla, M.; Tolosa de Talamoni, N. Vitamin D receptor genotypes are associated with bone mass in patients with Turner syndrome. J. Pediatr. Endocrinol. Metab. 2011, 24, 307–312. [Google Scholar] [CrossRef]
- Shi, K.; Liu, L.; He, Y.J.; Li, D.; Yuan, L.X.; Lash, G.E.; Li, L. Body composition and bone mineral status in patients with Turner syndrome. Sci. Rep. 2016, 6, 38026. [Google Scholar] [CrossRef]
- Wüster, C.; Härle, U.; Rehn, U.; Müller, C.; Knauf, K.; Köppler, D.; Schwabe, C.; Ziegler, R. Benefits of growth hormone treatment on bone metabolism, bone density and bone strength in growth hormone deficiency and osteoporosis. Growth Horm. IGF Res. 1998, 8, 87–94. [Google Scholar] [CrossRef]
- Allen, D.B.; Fost, N.C. Growth hormone therapy for short stature: Panacea or Pandora’s box? J. Pediatr. 1990, 117, 16–21. [Google Scholar] [CrossRef]
- Fisher, B.G.; Acerini, C.L. Understanding the growth hormone therapy adherence paradigm: A systematic review. Horm. Res. Paediatr. 2013, 79, 189–196. [Google Scholar] [CrossRef]
- Klein, K.O.; Rosenfield, R.L.; Santen, R.J.; Gawlik, A.M.; Backeljauw, P.F.; Gravholt, C.H.; Sas, T.C.J.; Mauras, N. Estrogen Replacement in Turner Syndrome: Literature Review and Practical Considerations. J. Clin. Endocrinol. Metab. 2018, 103, 1790–1803. [Google Scholar] [CrossRef]
- Souza, F.M.; Collett-Solberg, P.F. Adverse effects of growth hormone replacement therapy in children. Arq. Bras. Endocrinol. Metabol. 2011, 55, 559–565. [Google Scholar] [CrossRef]
- Alvarez-Nava, F.; Marcano, H.; Pardo, T.; Paoli, M.; Gunczler, P.; Soto, M.; Villaloboss, J.; Lanes, R. GHR and VDR genes do not contribute to the growth hormone (GH) response in GH deficient and Turner syndrome patients. J. Pediatr. Endocrinol. Metab. 2010, 23, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Miedziaszczyk, M.; Lacka, K.; Tomczak, O.; Bajon, A.; Primke, M.; Idasiak-Piechocka, I. Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation. Biomedicines 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Rios, R.; Frias, S.; Velázquez-Aragón, J.A.; Villaroel, C.E.; Sánchez, S.; Molina, B.; Martínez, A.; Carnevale, A.; García-de-Teresa, B.; Bonilla, E.; et al. Low bone mineral density and renal malformation in Mexican patients with Turner syndrome are associated with single nucleotide variants in vitamin D-metabolism genes. Gynecol. Endocrinol. 2019, 35, 772–776. [Google Scholar] [CrossRef]
- Choe, H.S.; Lee, J.H.; Min, D.K.; Shin, S.H. Comparison of vertebral and femoral bone mineral density in adult females. J. Phys. Ther. Sci. 2016, 28, 1928–1931. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Ge, B.F.; Liu, X.Y.; Ma, P.H.; Lu, M.B.; Bai, M.H.; Wang, Y. Icariin inhibits the osteoclast formation induced by RANKL and macrophage-colony stimulating factor in mouse bone marrow culture. Pharmazie 2007, 62, 388–391. [Google Scholar] [PubMed]
- Seok, H.; Kim, K.J.; Kim, K.M.; Rhee, Y.; Cha, B.S.; Lim, S.K. High prevalence of spine–femur bone mineral density discordance and comparison of vertebral fracture risk assessment using femoral neck and lumbar spine bone density in Korean patients. J. Bone Miner. Metab. 2014, 32, 405–410. [Google Scholar] [CrossRef]



| Author, Years | Total Population | Age (Years) | GHT (Number of Group Members) | BMD [g·cm−3] | Duration of ERT (Years) | Age of Start ERT (Years) | Height (cm) | |
|---|---|---|---|---|---|---|---|---|
| Before ERT | After ERT | |||||||
| Sowińska-Przepiera E., 2005 [15] | 34 | 22.7 ± 8.2 (12–39) | No treatment | 0.802 ± 0.134 | 0.951 ± 0.148 | 4 years | 22.7 ± 8.2 (12–39) | 141.7 ± 8.8 |
| Nakamura T., 2015 [16] | 21 (late initiation of ERT) | 35.5 ± 8.2 (26–58) | 11/21 | 0.743 ± 0.126 | 0.795 ± 0.107 | 13.5 ± 5.7 (7–27) | 19.9 ± 1.6 (18–22) | 144.7 ± 5.3 (132.0–151.0) |
| 16 (early initiation of ERT) | 28.8 ± 6.1 (18–40) | 8/16 | 0.809 ± 0.120 | 0.832 ± 0.119 | 12.8 ± 6.1 (1–23) | 16.6 ± 1.2 (14–17) | 146.2 ± 5.1 (134.0–151.0) | |
| Li L., 2019 [14] | 20 | 18.45 ± 3 (16–21) | No treatment | 0.69 ± 0.09 | 0.73 ± 0.09 | 1 year | 18.45 ± 3.07 (16–21) | 144.89 ± 8.77 (131.8–163) |
| Author, Year GHT Application | Total Population | Age (Years) | ERT | BMD [g·cm−3] | Duration of GHT (Years) | Height [cm] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHT + | GHT − | GHT + | GHT − | GHT + | GHT − | GHT + | GHT − | GHT + | GHT − | GHT + | GHT − | |
| Ari M., 2006 [1] | 39 | 28 | 11.9 ± 2.8 | 12.8 ± 3.1 | 12/39 (31%) | 6/28 (21%) | 0.67 ± 0.13 | 0.70 ± 0.15 | 4.2 ± 3.2 (1–14) | ----- | 137 ± 13.6 | 134 ± 13.9 |
| Bakalov V., 2004 [17] | 23 | 23 | 21.5 ± 9.4 (7–35) | 21.7 ± 9.4 (7–37) | 15/16 (94%) | 14/16 (89%) | 0.82 ± 0.14 | 0.80 ± 0.16 | 5.0 ± 2.1 | ----- | 145.2 ± 10.9 | 143.3 ± 12.3 |
| Nour M., 2016 [18] | 12 | 16 | 26.7 ± 6.9 | 28.3 ± 7.2 | + | + | 0.92 ± 0.16 | 0.89 ± 0.13 | median 5 (2–13) | ----- | 151.3 ± 6.3 | 143.9 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szybiak, W.; Kujawa, B.; Miedziaszczyk, M.; Lacka, K. Effect of Growth Hormone and Estrogen Replacement Therapy on Bone Mineral Density in Women with Turner Syndrome: A Meta-Analysis and Systematic Review. Pharmaceuticals 2023, 16, 1320. https://doi.org/10.3390/ph16091320
Szybiak W, Kujawa B, Miedziaszczyk M, Lacka K. Effect of Growth Hormone and Estrogen Replacement Therapy on Bone Mineral Density in Women with Turner Syndrome: A Meta-Analysis and Systematic Review. Pharmaceuticals. 2023; 16(9):1320. https://doi.org/10.3390/ph16091320
Chicago/Turabian StyleSzybiak, Weronika, Barbara Kujawa, Miłosz Miedziaszczyk, and Katarzyna Lacka. 2023. "Effect of Growth Hormone and Estrogen Replacement Therapy on Bone Mineral Density in Women with Turner Syndrome: A Meta-Analysis and Systematic Review" Pharmaceuticals 16, no. 9: 1320. https://doi.org/10.3390/ph16091320
APA StyleSzybiak, W., Kujawa, B., Miedziaszczyk, M., & Lacka, K. (2023). Effect of Growth Hormone and Estrogen Replacement Therapy on Bone Mineral Density in Women with Turner Syndrome: A Meta-Analysis and Systematic Review. Pharmaceuticals, 16(9), 1320. https://doi.org/10.3390/ph16091320