Effect of Supplementation with Omega-3 Polyunsaturated Fatty Acids on Metabolic Modulators in Skeletal Muscle of Rats with an Obesogenic High-Fat Diet
Abstract
:1. Introduction
2. Results
2.1. The Characteristics in the Obese Model Induced by a High-Fat Diet
2.2. Effect of Omega-3 Polyunsaturated Fatty Acids
2.2.1. Zoometric Parameters and Weight of Skeletal Muscle (Soleus)
2.2.2. Biochemical Profile, Adiponectin, and Insulin
2.2.3. Glucose (GTT) and Insulin Tolerance Tests (ITT)
2.2.4. Metabolism Modulators in Skeletal Muscle
3. Discussion
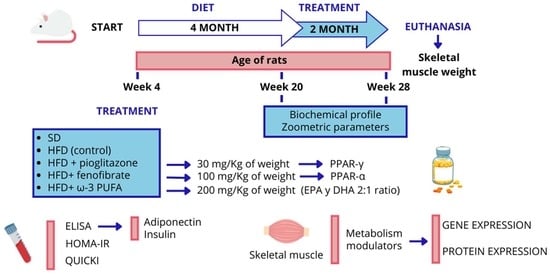
4. Materials and Methods
4.1. Animal Model
4.2. Zoometric Parameters and Biochemical Profile
4.3. Glucose Tolerance Test
4.4. Insulin Tolerance Test
4.5. Euthanasia and Dissection
4.6. Serum Concentration of Adiponectin, Insulin, and Determination of HOMA-IR and QUICKI
4.7. Real-Time Polymerase Chain Reaction (qPCR)
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | The area under the curve |
| GLUT-4 | Glucose transporter 4 |
| GPR120 | G protein-coupled receptor 120 |
| GTT | Glucose tolerance test |
| HCl | Hydrochloric acid |
| HFD | High-fat diet |
| HOMA-IR | Homeostatic model assessment |
| INSR | Insulin receptor |
| ISR-1 | Insulin Receptor Substrate 1 |
| ITT | Insulin tolerance test |
| Kitt | Constant for glucose disappearance rate |
| LPL | Lipoprotein lipase |
| PI3K | Phosphatidylinositol-3-kinase |
| PIK3CA | Phosphatidylinositol-3-kinase alpha catalytic subunit |
| PPARs | Peroxisome proliferator-activated receptor |
| PPAR-α | Peroxisome proliferator-activated receptor alpha |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| QUICKI | Quantitative insulin sensitivity check index |
| RNA | Ribonucleic acid |
| SD | Standard diet |
| SDS | Sodium dodecyl sulphate |
| SREBP-1c | Sterol regulatory element binding protein expression |
| VLDL | Very low-density lipoproteins |
| ω-3 PUFA | Omega-3 polyunsaturated fatty acids |
References
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. 2020. Available online: http://origin.who.int (accessed on 15 July 2023).
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://www.worldobesity.org/ (accessed on 7 August 2023).
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Nguyet, N.T.M.; Dinh, T.C.; Lien, N.V.T.; Nguyen, K.H.; Ngoc, V.T.N.; Tao, Y.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPARα), beta (PPARβ), and gamma (PPARγ) in rodent and human development. Reprod. Toxicol. 2009, 27, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.; Zhuang, Y.; Wahli, W. Synthetic and natural Peroxisome Proliferator-Activated Receptor (PPAR) agonists as candidates for the therapy of the metabolic syndrome. Expert Opin. Ther. Targets 2017, 21, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Phua, W.W.T.; Wong, M.X.Y.; Liao, Z.; Tan, N.S. An aPPARent Functional Consequence in Skeletal Muscle Physiology via Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2018, 19, 1425. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association with Metabolic Risk Factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Guo, S. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Tan, M. Tiazolidinadionas: Efecto de la pioglitazona sobre la hiperglucemia, la Dislipidemia y el riesgo cardiovascular. Rev. Clínica Española 2003, 203, 33–40. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.-A. Is There a Role for Fibrates in the Management of Dyslipidemia in the Metabolic Syndrome? Arter. Thromb. Vasc. Biol. 2008, 28, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, X.-B.; Liu, Y.-C.; Yu, W.-Q.; Si, Y.-H.; Guo, S.-D. Fenofibrate enhances lipid deposition via modulating PPARγ, SREBP-1c, and gut microbiota in ob/ob mice fed a high-fat diet. Front. Nutr. 2022, 9, 971581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F. N-3 Polyunsaturated fatty acids and inflammation in obesity: Local effect and systemic benefit. BioMed Res. Int. 2015, 2015, 581469. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.J.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010, 140, 1915–1922. [Google Scholar] [CrossRef]
- Pahlavani, M.; Ramalho, T.; Koboziev, I.; LeMieux, M.J.; Jayarathne, S.; Ramalingam, L.; Filgueiras, L.R.; Moustaid-Moussa, N. Adipose tissue inflammation in insulin resistance: Review of mechanisms mediating anti-inflammatory effects of omega-3 polyunsaturated fatty acids. J. Investig. Med. 2017, 65, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation. iScience 2022, 25, 104869. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Filho, J.L.V.B.N. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Festuccia, W.T.; Crisma, A.R.; Alves, V.S.; Martins, A.R.; Amaral, C.L.; Fiamoncini, J.; Hirabara, S.M.; Sato, F.T.; et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E272–E282. [Google Scholar] [CrossRef]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.d.O.; Pereira, S.S.; de Oliveira, L.L.; Peluzio, M.D.C.G.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Quon, M.J. Assessing the Predictive Accuracy of QUICKI as a Surrogate Index for Insulin Sensitivity Using a Calibration Model. Diabetes 2005, 54, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Almeda-Valdés, P.; Bello-Chavolla, O.Y.; Caballeros-Barragán, C.R.; Gómez-Velasco, D.V.; Viveros-Ruiz, T.; Vargas-Vázquez, A.; Aguilar-Salinas, C.A. Índices para la evaluación de la resistencia a la insulina en individuos mexicanos sin diabetes. Gac. Medica De Mex. 2018, 154, S50–S55. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.M.; Stein, D.J.; Medeiros, H.R.; de Oliveira, C.; Torres, I.L.S. Nicotinamide Riboside Neutralizes Hypothalamic Inflammation and Increases Weight Loss without Altering Muscle Mass in Obese Rats Under Calorie Restriction: A Preliminary Investigation. Front. Nutr. 2021, 8, 648893. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Mobarhan, M.G.; Rezaiean, S.; Hoseini, M.; Parizade, S.; Farhoudi, F.; Hosseininezhad, S.; Tavallaei, S.; Vejdani, A.; Azimi-Nezhad, M.; et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009, 64, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.A.; Garg, M.L. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obes. Res. Clin. Pract. 2013, 7, e173–e181. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; O Kouba, E.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, L.; de Oliveira, E.P.; Moreto, F.; Portero-McLellan, K.C.; Burini, R.C. Omega-3 fatty acids supplementation decreases metabolic syndrome prevalence after lifestyle modification program. J. Funct. Foods 2015, 19, 922–928. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yahagi, N.; Izumida, Y.; Nishi, M.; Kubota, M.; Teraoka, Y.; Yamamoto, T.; Matsuzaka, T.; Nakagawa, Y.; Sekiya, M.; et al. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit. J. Biol. Chem. 2010, 285, 11681–11691. [Google Scholar] [CrossRef]
- Chadli, F.K.; Andre, A.; Prieur, X.; Loirand, G.; Meynier, A.; Krempf, M.; Nguyen, P.; Ouguerram, K. n-3 PUFA prevent metabolic disturbances associated with obesity and improve endothelial function in golden Syrian hamsters fed with a high-fat diet. Br. J. Nutr. 2012, 107, 1305–1315. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Omega-3 polyunsaturated fatty acids in the treatment of atherogenic dyslipidemia. Atheroscler. Suppl. 2013, 14, 237–242. [Google Scholar] [CrossRef] [PubMed]
- A Jacobson, T. Role of n−3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am. J. Clin. Nutr. 2008, 87, 1981S–1990S. [Google Scholar] [CrossRef] [PubMed]
- Ebbesson, S.O.E.; Risica, P.M.; Ebbesson, L.O.E.; Kennish, J.M.; Tejero, M.E. Omega-3 fatty acids improve glucose tolerance and components of the metabolic syndrome in Alaskan Eskimos: The Alaska Siberia project. Int. J. Circumpolar Health 2005, 64, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Del-Río-Navarro, B.E.; Leija-Martinez, J.; Torres-Alcantara, S.; Ruiz-Bedolla, E.; Hernández-Cadena, L.; Barraza-Villarreal, A.; Romero-Nava, R.; Sanchéz-Muñoz, F.; Villafaña, S.; et al. Effect of omega-3 fatty acids supplementation combined with lifestyle intervention on adipokines and biomarkers of endothelial dysfunction in obese adolescents with hypertriglyceridemia. J. Nutr. Biochem. 2019, 64, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Taouis, M.; Dagou, C.; Ster, C.; Durand, G.; Pinault, M.; Delarue, J. n-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am. J. Physiol. Metab. 2002, 282, E664–E671. [Google Scholar] [CrossRef]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic Restoration of Long-Chain n-3 Fatty Acids in Insulin Target Tissues Improves Resolution Capacity and Alleviates Obesity-Linked Inflammation and Insulin Resistance in High-Fat–Fed Mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef]
- Franck, M.; de Toro-Martín, J.; Guénard, F.; Rudkowska, I.; Lemieux, S.; Lamarche, B.; Couture, P.; Vohl, M.-C. Prevention of Potential Adverse Metabolic Effects of a Supplementation with Omega-3 Fatty Acids Using a Genetic Score Approach. Lifestyle Genom. 2019, 13, 32–42. [Google Scholar] [CrossRef]
- López-Alarcón, M.; Martínez-Coronado, A.; Velarde-Castro, O.; Rendón-Macías, E.; Fernández, J. Supplementation of n3 Long-chain Polyunsaturated Fatty Acid Synergistically Decreases Insulin Resistance with Weight Loss of Obese Prepubertal and Pubertal Children. Arch. Med. Res. 2011, 42, 502–508. [Google Scholar] [CrossRef]
- Kabir, M.; Skurnik, G.; Naour, N.; Pechtner, V.; Meugnier, E.; Rome, S.; Quignard-Boulangé, A.; Vidal, H.; Slama, G.; Clément, K.; et al. Treatment for 2 mo with n−3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: A randomized controlled study. Am. J. Clin. Nutr. 2007, 86, 1670–1679. [Google Scholar] [CrossRef]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet–induced obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Czepulis, N.; Lewandowicz, M.; Zasadzka, E.; Suwalska, A.; Witowski, J.; Wieczorowska-Tobis, K. The Effect of a 12-Week Omega-3 Supplementation on Body Composition, Muscle Strength and Physical Performance in Elderly Individuals with Decreased Muscle Mass. Int. J. Environ. Res. Public Health 2015, 12, 10558–10574. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Constantin-Teodosiu, D.; Greenhaff, P.L. The Regulatory Roles of PPARs in Skeletal Muscle Fuel Metabolism and Inflammation: Impact of PPAR Agonism on Muscle in Chronic Disease, Contraction and Sepsis. Int. J. Mol. Sci. 2021, 22, 9775. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic Acid–Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) With Anti-inflammatory Properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Bjursell, M.; Xu, X.; Admyre, T.; Böttcher, G.; Lundin, S.; Nilsson, R.; Stone, V.M.; Morgan, N.G.; Lam, Y.Y.; Storlien, L.H.; et al. The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS ONE 2014, 9, e114942. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPARγ Mediates High-Fat Diet–Induced Adipocyte Hypertrophy and Insulin Resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Wagener, A.; Goessling, H.F.; O Schmitt, A.; Mauel, S.; Gruber, A.D.; Reinhardt, R.; A Brockmann, G. Genetic and diet effects on Ppar-α and Ppar-γ signaling pathways in the Berlin Fat Mouse Inbred line with genetic predisposition for obesity. Lipids Health Dis. 2010, 9, 99. [Google Scholar] [CrossRef]
- Gani, O.A. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Veleba, J.; Kopecky, J., Jr.; Janovska, P.; Kuda, O.; Horakova, O.; Malinska, H.; Kazdova, L.; Oliyarnyk, O.; Skop, V.; Trnovska, J.; et al. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: Improvement of lipid metabolism. Nutr. Metab. 2015, 12, 52. [Google Scholar] [CrossRef] [PubMed]








| SD | HFD | |
|---|---|---|
| Initial body weight (g) | 123.1 ± 2.81 | 109.1 ± 6.06 |
| Final body weight (g) | 441.5 ± 7.26 | 545.1 ± 6.98 * |
| Abdominal circumference (cm) | 19.0 ± 0.17 | 21.0 ± 0.13 * |
| Index lee | 0.356 ± 0.001 | 0.366 ± 0.0008 * |
| Triglycerides (mg/dL) | 105.6 ± 2.50 | 124.9 ± 1.40 * |
| Glucose (mg/dL) | 82.4 ± 1.63 | 107.7 ± 1.38 * |
| Cholesterol (mg/dL) | 158.8 ± 2.10 | 164.5 ± 0.82 |
| SD | HFD | HFD + ω-3PUFA | HFD + FENO | HFD + PIO | |
|---|---|---|---|---|---|
| Body weight (g) | 479.8 ± 7.2 | 649.3 ± 29.73 * | 651.80 ± 17.12 | 617.1 ± 30.05 | 636.2 ± 27.90 |
| Abdominal circumference (cm) | 19.5 ± 0.17 | 22.8 ± 0.66 * | 22.1 ± 0.18 | 21.5 ± 0.17 | 22.2 ± 0.22 |
| Index lee | 0.359 ± 0.0008 | 0.373 ± 0.0008 * | 0.370 ± 0.0004 | 0.369 ± 0.0004 | 0.373 ± 0.0013 |
| Triglycerides (mg/dL) | 117.6 ± 2.90 | 166.0 ± 2.59 * | 106.6 ± 2.23 # | 105.8 ± 5.36 # | 110.0 ± 3.84 # |
| Glucose (mg/dL) | 92.0 ± 3.08 | 121.60 ± 3.23 * | 92.00 ± 5.35 # | 86.2 ± 3.08 # | 84.0 ± 2.70 # |
| Cholesterol (mg/dL) | 163.6 ± 2.01 | 166.8 ± 1.15 | 161.4 ± 1.85 | 159.2 ± 2.41 # | 166.0 ± 1.11 |
| Gene | Primer Sequence (5′–3′) | Gene Bank |
|---|---|---|
| Insr | F: TCAGAACCCGATGACCCTAC R: GGGATGCACTTGTTGTTGTG | NM_017071.2 |
| Irs-1 | F: GCTCTAGTGCTTCCGTGTCC R: GTTGCCACCCCTAGACAAAA | NM_012969.2 |
| Pi3k-Ca | F: CATCAGTGGCTCAAGGACAA R: CAGCTGTCCGTCATCTTTCA | NM_133399.3 |
| Glut-4 | F: CCTCCAGGATGAAGGAAACA R: GGGTAAGAGGAAGGCAGGAC | NM_012751.1 |
| Ppar-α, | F: CTCGTGCAGGTCATCAAGAA R: CAGCCCTCTTCATCTCCAAG | NM_013196.2 |
| Ppar-γ | F: CTGGCCTCCCTGATGAATAA R: GGCGGTCTCCACTGAGAATA | NM_001145366.1 |
| Gapdh | F: AGACAGCCGCATCTTCTTGT R: TTCCCATTC TCAGCCTTGAC | NM_017008.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Ortega, M.P.; Almanza-Pérez, J.C.; Sánchez-Muñoz, F.; Hong, E.; Velázquez-Reyes, E.; Romero-Nava, R.; Villafaña-Rauda, S.; Pérez-Ontiveros, A.; Blancas-Flores, G.; Huang, F. Effect of Supplementation with Omega-3 Polyunsaturated Fatty Acids on Metabolic Modulators in Skeletal Muscle of Rats with an Obesogenic High-Fat Diet. Pharmaceuticals 2024, 17, 222. https://doi.org/10.3390/ph17020222
Chávez-Ortega MP, Almanza-Pérez JC, Sánchez-Muñoz F, Hong E, Velázquez-Reyes E, Romero-Nava R, Villafaña-Rauda S, Pérez-Ontiveros A, Blancas-Flores G, Huang F. Effect of Supplementation with Omega-3 Polyunsaturated Fatty Acids on Metabolic Modulators in Skeletal Muscle of Rats with an Obesogenic High-Fat Diet. Pharmaceuticals. 2024; 17(2):222. https://doi.org/10.3390/ph17020222
Chicago/Turabian StyleChávez-Ortega, Mara Patricia, Julio Cesar Almanza-Pérez, Fausto Sánchez-Muñoz, Enrique Hong, Elihu Velázquez-Reyes, Rodrigo Romero-Nava, Santiago Villafaña-Rauda, Alfredo Pérez-Ontiveros, Gerardo Blancas-Flores, and Fengyang Huang. 2024. "Effect of Supplementation with Omega-3 Polyunsaturated Fatty Acids on Metabolic Modulators in Skeletal Muscle of Rats with an Obesogenic High-Fat Diet" Pharmaceuticals 17, no. 2: 222. https://doi.org/10.3390/ph17020222
APA StyleChávez-Ortega, M. P., Almanza-Pérez, J. C., Sánchez-Muñoz, F., Hong, E., Velázquez-Reyes, E., Romero-Nava, R., Villafaña-Rauda, S., Pérez-Ontiveros, A., Blancas-Flores, G., & Huang, F. (2024). Effect of Supplementation with Omega-3 Polyunsaturated Fatty Acids on Metabolic Modulators in Skeletal Muscle of Rats with an Obesogenic High-Fat Diet. Pharmaceuticals, 17(2), 222. https://doi.org/10.3390/ph17020222