Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study
Abstract
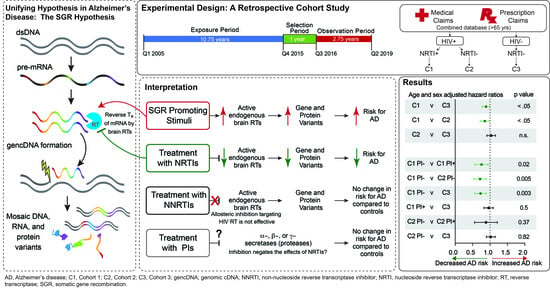
:1. Introduction
2. Results
2.1. Study Participant Characteristics
2.2. Antiretroviral Exposure and New AD Incidence
3. Discussion
4. Materials and Methods
4.1. Data Sources, Study Population and Selection of Cohorts
4.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaugler, J.; James, B.; Johnson, T.; Reimer, J.; Solis, M.; Weuve, J.; Hohman, T.J. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Lee, M.H.; Siddoway, B.; Kaeser, G.E.; Segota, I.; Rivera, R.; Romanow, W.J.; Liu, C.S.; Park, C.; Kennedy, G.; Long, T.; et al. Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nature 2018, 563, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, G.E.; Chun, J. Mosaic Somatic Gene Recombination as a Potentially Unifying Hypothesis for Alzheimer’s Disease. Front Genet. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chun, J. Mosaic APP Gene Recombination in Alzheimer’s Disease-What’s Next? J. Exp. Neurosci. 2019, 13, 1179069519849669. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, J.; Jung, E.S.; Kim, M.H.; Kim, I.B.; Son, H.; Kim, S.; Kim, S.; Park, Y.M.; Mook-Jung, I.; et al. Brain somatic mutations observed in Alzheimer’s disease associated with aging and dysregulation of tau phosphorylation. Nat. Commun. 2019, 10, 3090. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.R.; Liu, C.S.; Romanow, W.J.; Lee, M.H.; Chun, J. Altered cell and RNA isoform diversity in aging Down syndrome brains. Proc. Natl. Acad. Sci. USA 2021, 118, e2114326118. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Fujito, N.; Nakaoka, H.; Imazeki, R.; Nagata, E.; Inoue, I. Detection of APP gene recombinant in human blood plasma. Sci. Rep. 2023, 13, 21703. [Google Scholar] [CrossRef]
- Rovelet-Lecrux, A.; Hannequin, D.; Raux, G.; Le Meur, N.; Laquerriere, A.; Vital, A.; Dumanchin, C.; Feuillette, S.; Brice, A.; Vercelletto, M.; et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006, 38, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Sleegers, K.; Brouwers, N.; Gijselinck, I.; Theuns, J.; Goossens, D.; Wauters, J.; Del-Favero, J.; Cruts, M.; van Duijn, C.M.; Van Broeckhoven, C. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 2006, 129, 2977–2983. [Google Scholar] [CrossRef]
- Prasher, V.P.; Farrer, M.J.; Kessling, A.M.; Fisher, E.M.; West, R.J.; Barber, P.C.; Butler, A.C. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 1998, 43, 380–383. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Bird, E.D.; Latt, S.A.; Neve, R.L. The amyloid beta protein gene is not duplicated in brains from patients with Alzheimer’s disease. Science 1987, 238, 666–669. [Google Scholar] [CrossRef] [PubMed]
- St George-Hyslop, P.H.; Tanzi, R.E.; Polinsky, R.J.; Neve, R.L.; Pollen, D.; Drachman, D.; Growdon, J.; Cupples, L.A.; Nee, L.; Myers, R.H.; et al. Absence of duplication of chromosome 21 genes in familial and sporadic Alzheimer’s disease. Science 1987, 238, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Bushman, D.M.; Kaeser, G.E.; Siddoway, B.; Westra, J.W.; Rivera, R.R.; Rehen, S.K.; Yung, Y.C.; Chun, J. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer’s disease brains. eLife 2015, 4, e05116. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (Updated); Volume 31. Available online: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-31/index.html (accessed on 9 November 2023).
- Turner, R.S.; Chadwick, M.; Horton, W.A.; Simon, G.L.; Jiang, X.; Esposito, G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Alisky, J.M. The coming problem of HIV-associated Alzheimer’s disease. Med. Hypotheses 2007, 69, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ikezu, T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: A foreseeable medical challenge in post-HAART era. J. Neuroimmune Pharmacol. 2009, 4, 200–212. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Morris, C.R.; Liu, L.; Bungum, T.J.; Altekruse, S.F. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J. Natl. Cancer Inst. Monogr. 2014, 2014, 210–217. [Google Scholar] [CrossRef]
- Baldwin, E.T.; van Eeuwen, T.; Hoyos, D.; Zalevsky, A.; Tchesnokov, E.P.; Sanchez, R.; Miller, B.D.; Di Stefano, L.H.; Ruiz, F.X.; Hancock, M.; et al. Structures, functions and adaptations of the human LINE-1 ORF2 protein. Nature 2024, 626, 194–206. [Google Scholar] [CrossRef]
- Thawani, A.; Ariza, A.J.F.; Nogales, E.; Collins, K. Template and target-site recognition by human LINE-1 in retrotransposition. Nature 2024, 626, 186–193. [Google Scholar] [CrossRef]
- Baldwin, E.T.; Gotte, M.; Tchesnokov, E.P.; Arnold, E.; Hagel, M.; Nichols, C.; Dossang, P.; Lamers, M.; Wan, P.; Steinbacher, S.; et al. Human endogenous retrovirus-K (HERV-K) reverse transcriptase (RT) structure and biochemistry reveals remarkable similarities to HIV-1 RT and opportunities for HERV-K-specific inhibition. Proc. Natl. Acad. Sci. USA 2022, 119, e2200260119. [Google Scholar] [CrossRef]
- Fowler, B.J.; Gelfand, B.D.; Kim, Y.; Kerur, N.; Tarallo, V.; Hirano, Y.; Amarnath, S.; Fowler, D.H.; Radwan, M.; Young, M.T.; et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014, 346, 1000–1003. [Google Scholar] [CrossRef]
- Kong, H.; Zhao, H.; Chen, T.; Song, Y.; Cui, Y. Targeted P2 × 7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 2022, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kaneko, H.; Shimizu, H.; Suzumura, A.; Namba, R.; Takayama, K.; Ito, S.; Sugimoto, M.; Terasaki, H. Lamivudine Inhibits Alu RNA-induced Retinal Pigment Epithelium Degeneration via Anti-inflammatory and Anti-senescence Activities. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Saresella, M.; Marventano, I.; Piancone, F.; Ripamonti, E.; Al-Daghri, N.; Bazzini, C.; Zoia, C.P.; Conti, E.; Ferrarese, C.; et al. Stavudine Reduces NLRP3 Inflammasome Activation and Modulates Amyloid-beta Autophagy. J. Alzheimers Dis. 2019, 72, 401–412. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Zoia, C.P.; Bazzini, C.; Bolognini, A.; Saresella, M.; Conti, E.; Ferrarese, C.; Piancone, F.; Marventano, I.; Galimberti, D.; et al. Modulation of MAPK- and PI3/AKT-Dependent Autophagy Signaling by Stavudine (D4T) in PBMC of Alzheimer’s Disease Patients. Cells 2022, 11, 2180. [Google Scholar] [CrossRef] [PubMed]
- Valles-Saiz, L.; Avila, J.; Hernandez, F. Lamivudine (3TC), a Nucleoside Reverse Transcriptase Inhibitor, Prevents the Neuropathological Alterations Present in Mutant Tau Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 11144. [Google Scholar] [CrossRef]
- Wahl, D.; Smith, M.E.; McEntee, C.M.; Cavalier, A.N.; Osburn, S.C.; Burke, S.D.; Grant, R.A.; Nerguizian, D.; Lark, D.S.; Link, C.D.; et al. The reverse transcriptase inhibitor 3TC protects against age-related cognitive dysfunction. Aging Cell 2023, 22, e13798. [Google Scholar] [CrossRef]
- Martinez de Lagran, M.; Elizalde-Torrent, A.; Paredes, R.; Clotet, B.; Dierssen, M. Lamivudine, a reverse transcriptase inhibitor, rescues cognitive deficits in a mouse model of down syndrome. J. Cell Mol. Med. 2022, 26, 4210–4215. [Google Scholar] [CrossRef]
- Haass, C.; Steiner, H. Alzheimer disease gamma-secretase: A complex story of GxGD-type presenilin proteases. Trends Cell Biol. 2002, 12, 556–562. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, R.; Yang, G.; Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc. Natl. Acad. Sci. USA 2017, 114, E476–E485. [Google Scholar] [CrossRef]
- De Strooper, B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007, 8, 141–146. [Google Scholar] [CrossRef]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data—United States and 6 Dependent Areas, 2017. HIV Surveillance Supplemental Report 2019; 24 (No. 3). Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 9 November 2023).
- Brew, B.J.; Pemberton, L.; Blennow, K.; Wallin, A.; Hagberg, L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology 2005, 65, 1490–1492. [Google Scholar] [CrossRef]
- Siangphoe, U.; Archer, K.J.; Nguyen, C.; Lee, K.R. Associations of antiretroviral therapy and comorbidities with neurocognitive outcomes in HIV-1-infected patients. AIDS 2020, 34, 893–902. [Google Scholar] [CrossRef]
- Aung, H.L.; Bloch, M.; Vincent, T.; Mao, L.; Brew, B.J.; Cysique, L.A. Low incidence of advanced neurological burden but high incidence of age-related conditions that are dementia risk factors in aging people living with HIV: A data-linkage 10-year follow-up study. J. Neurovirol. 2022, 29, 141–155. [Google Scholar] [CrossRef]
- Kern, D.M.; Cepeda, M.S.; Lovestone, S.; Seabrook, G.R. Aiding the discovery of new treatments for dementia by uncovering unknown benefits of existing medications. Alzheimers Dement. 2019, 5, 862–870. [Google Scholar] [CrossRef]
- Gelman, B.B. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr. HIV/AIDS Rep. 2015, 12, 272–279. [Google Scholar] [CrossRef]
- Gougeon, M.L.; Poirier-Beaudouin, B.; Durant, J.; Lebrun-Frenay, C.; Saidi, H.; Seffer, V.; Ticchioni, M.; Chanalet, S.; Carsenti, H.; Harvey-Langton, A.; et al. HMGB1/anti-HMGB1 antibodies define a molecular signature of early stages of HIV-Associated Neurocognitive Isorders (HAND). Heliyon 2017, 3, e00245. [Google Scholar] [CrossRef]
- Anderson, S.G.; McCaul, M.; Khoo, S.; Wiesner, L.; Sacktor, N.; Joska, J.A.; Decloedt, E.H. The neurologic phenotype of South African patients with HIV-associated neurocognitive impairment. Neurol. Clin. Pract. 2020, 10, 15–22. [Google Scholar] [CrossRef]
- Gisslen, M.; Krut, J.; Andreasson, U.; Blennow, K.; Cinque, P.; Brew, B.J.; Spudich, S.; Hagberg, L.; Rosengren, L.; Price, R.W.; et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009, 9, 63. [Google Scholar] [CrossRef]
- Peterson, J.; Gisslen, M.; Zetterberg, H.; Fuchs, D.; Shacklett, B.L.; Hagberg, L.; Yiannoutsos, C.T.; Spudich, S.S.; Price, R.W. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: Hierarchy of injury and detection. PLoS ONE 2014, 9, e116081. [Google Scholar] [CrossRef]
- Cooley, S.A.; Nelson, B.; Boerwinkle, A.; Yarasheski, K.E.; Kirmess, K.M.; Meyer, M.R.; Schindler, S.E.; Morris, J.C.; Fagan, A.; Ances, B.M.; et al. Plasma Abeta42/Abeta40 ratios are not reduced in older people living with HIV. Clin. Infect. Dis. 2023, 76, 1776–1783. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Umlauf, A.; Soontornniyomkij, B.; Gouaux, B.; Ellis, R.J.; Levine, A.J.; Moore, D.J.; Letendre, S.L. Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS 2018, 32, 2005–2015. [Google Scholar] [CrossRef]
- Davidson, Y.S.; Robinson, A.; Prasher, V.P.; Mann, D.M.A. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathol. Commun. 2018, 6, 56. [Google Scholar] [CrossRef]
- Rabano, A.; Jimenez-Huete, A.; Acevedo, B.; Calero, M.; Ghiso, J.; Valdes, I.; Gavilondo, J.; Frangione, B.; Mendez, E. Diversity of senile plaques in Alzheimer’s disease as revealed by a new monoclonal antibody that recognizes an internal sequence of the Abeta peptide. Curr. Alzheimer Res. 2005, 2, 409–417. [Google Scholar] [CrossRef]
- Juday, T.; Grimm, K.; Zoe-Powers, A.; Willig, J.; Kim, E. A retrospective study of HIV antiretroviral treatment persistence in a commercially insured population in the United States. AIDS Care 2011, 23, 1154–1162. [Google Scholar] [CrossRef]
- Woodring, J.; Kruszon-Moran, D.; McQuillan, G. HIV Infection in U.S. Household Population Aged 18-59: Data from the National Health and Nutrition Examination Survey, 2007-2012. Natl. Health Stat Rep. 2015, 83, 1–13. [Google Scholar]
- Althoff, K.N.; Stewart, C.N.; Humes, E.; Zhang, J.; Gerace, L.; Boyd, C.M.; Wong, C.; Justice, A.C.; Gebo, K.A.; Thorne, J.E.; et al. The shifting age distribution of people with HIV using antiretroviral therapy in the United States. AIDS 2022, 36, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.; Rosa-Neto, P. World Alzheimer Report 2022: Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; Evans, D.A. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Antoniou, T.; Zagorski, B.; Loutfy, M.R.; Strike, C.; Glazier, R.H. Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. PLoS ONE 2011, 6, e21748. [Google Scholar] [CrossRef]



| Cohort 1 HIV+/NRTI+ N = 46,218 | Cohort 2 HIV+/NRTI− N = 32,923 | Cohort 3 HIV−/NRTI− N = 150,819 | |
|---|---|---|---|
| Median Age, yrs a | 64 | 65 | 69 b |
| Men, N (%) c | 34,226 (74.1%) | 22,407 (68.1%) | 57,336 d (38.3%) |
| History of IV drug-use, N (%) | 1990 (4.3%) | 1221 (3.7%) | 708 (0.47%) |
| Hemophilia, N (%) | 105 (0.2%) | 44 (0.1%) | 84 (0.06%) |
| Cohort 1 HIV+/NRTI+ N = 48,571 | Cohort 2 HIV+/NRTI− N = 37,252 | |
|---|---|---|
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) N (%, median duration in yrs) | ||
| emtricitabine/tenofovir disoproxil fumarate | 28,455 (61.6%, 4) | 0 (0.0%, 0) |
| abacavir sulfate/lamivudine | 13,256 (28.7%, 4) | 0 (0.0%, 0) |
| tenofovir disoproxil fumarate | 12,281 (26.6%, 3) | 0 (0.0%, 0) |
| lamivudine | 10,901 (23.6%, 2) | 0 (0.0%, 0) |
| abacavir | 7134 (15.4%, 3) | 0 (0.0%, 0) |
| didanosine | 4392 (9.5%, 3) | 0 (0.0%, 0) |
| Abacavir sulfate/lamivudine/zidovudine | 4085 (8.8%, 3) | 0 (0.0%, 0) |
| stavudine | 3754 (8.1%, 2) | 0 (0.0%, 0) |
| zidovudine | 2689 (5.8%, 2) | 0 (0.0%, 0) |
| emtricitabine | 2693 (5.8%, 2) | 0 (0.0%, 0) |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) N (%, median duration (yrs)) | ||
| efavirenz | 11,239 (24.3%, 3) | 1997 (6.1%, 4) |
| nevirapine | 5857 (12.7%, 5) | 703 (2.1%, 5) |
| etravirine | 4774 (10.3%, 4) | 443 (1.4%, 3) |
| rilpivirine | 1292 (2.8%, 2) | 64 (0.2%, 2) |
| delavirdine | 223 (0.5%, 3) | 15 (0.1%, 6) |
| Protease Inhibitors (PIs) N (%, median duration (yrs)) | ||
| ritonavir | 23,844 (51.6%, 4) | 1362 (4.1%, 3) |
| atazanavir | 14,808 (32.0%, 4) | 602 (1.8%, 4) |
| darunavir | 12,111 (26.2%, 4) | 786 (2.4%, 3) |
| lopinavir/ritonavir | 10,283 (22.3%, 3) | 816 (2.5%, 4) |
| fosamprenavir | 4362 (9.4%, 3) | 169 (0.5%, 4) |
| nelfinavir | 2563 (5.6%, 3) | 490 (1.5%, 5) |
| darunavir/cobicistat | 2067 (4.5%, 1) | 165 (0.5%, 1) |
| indinavir | 1072 (2.3%, 2) | 290 (0.9%, 4) |
| saquinavir | 1392 (3.0%, 3) | 153 (0.5%, 4) |
| tipranavir | 641 (1.4%, 2) | 12 (0.04%, 2) |
| atazanavir/cobicistat | 468 (1.0%, 1) | 18 (0.1%, 4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, T.W.; Raupp, M.; Reynolds, M.W.; Li, S.; Kaeser, G.E.; Chun, J. Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study. Pharmaceuticals 2024, 17, 408. https://doi.org/10.3390/ph17040408
Chow TW, Raupp M, Reynolds MW, Li S, Kaeser GE, Chun J. Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study. Pharmaceuticals. 2024; 17(4):408. https://doi.org/10.3390/ph17040408
Chicago/Turabian StyleChow, Tiffany W., Mark Raupp, Matthew W. Reynolds, Siying Li, Gwendolyn E. Kaeser, and Jerold Chun. 2024. "Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study" Pharmaceuticals 17, no. 4: 408. https://doi.org/10.3390/ph17040408
APA StyleChow, T. W., Raupp, M., Reynolds, M. W., Li, S., Kaeser, G. E., & Chun, J. (2024). Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study. Pharmaceuticals, 17(4), 408. https://doi.org/10.3390/ph17040408