Bench to Bedside: Stability Studies of GMP Produced Trastuzumab-TCMC in Support of a Clinical Trial
Abstract
:1. Introduction
2. Results
2.1. Visual Inspection
2.2. Integrity of cGMP TCMC-Trastuzumab
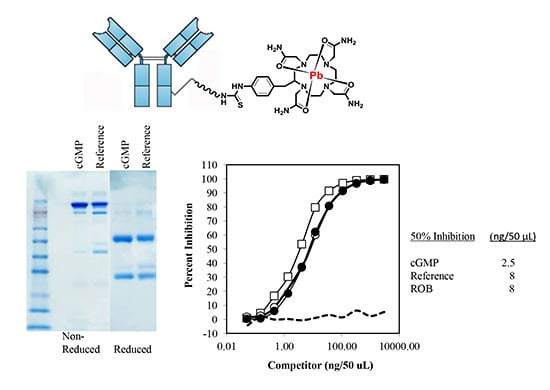
2.2.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)

2.2.2. Size-Exclusion High Performance Liquid Chromatography (SE-HPLC)

2.2.3. Ion-Exchange High Performance Liquid Chromatography (IEX-HPLC)

2.3. cGMP TCMC Radiolabeling and Analysis of Immunoreactivity Before and After Radiolabeling
2.3.1. In Vitro Analysis of cGMP 212Pb-TCMC-Trastuzumab
| Months | % Efficiency | % Free Chelate | Specific Activity (mCi/mg) | % Purity (SE-HPLC) |
|---|---|---|---|---|
| 0 | 92.2 | 0.6 | 3.2 | 100.0 |
| 3 | 92.2 | 0.2 | 3.3 | ND |
| 6 | 94.8 | 0.1 | 2.8 | 100.0 |
| 12 | 99.6 | 0.4 | 3.2 | 100.0 |
| 18 | 99.6 | 0.4 | 4.6 | 100.0 |
| 24 | 98.0 | 0.4 | 4.0 | 91.5 |
| 36 | 98.8 | 0.4 | 5.1 | 100.0 |
| 48 | 93.5 | 0.6 | 3.1 | 100.0 |
| 60 | 98.2 | 1.6 | 6.0 | 90.2 |
2.3.2. Immunoreactivity of cGMP TCMC-Trastuzumab
| Months | Competition | Immunoreactivity (% Bound) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Specific | Non-Specific | Specific | Non-Specific | Adjusted % Bound | |||||
| GMP | Ref | ROB | GMP | GMP | Ref | Ref | GMP | Ref | |
| 0 | 18.0 a | 20 | ND b | 73.1 | 4.8 | 72.8 | 3.0 | 68.3 c | 69.8 |
| 3 | 3.0 | 3.2 | ND | 76.2 | 10.8 | 75.2 | 14.7 | 65.4 | 60.5 |
| 6 | 2.6 | 3.1 | ND | 57.8 | 12.6 | 67.5 | 11.4 | 45.2 | 56.1 |
| 12 | 1.2 | 2.9 | 2.9 | 62.2 | 6.4 | 59.9 | 3.2 | 55.8 | 56.7 |
| 18 | 1.5 | 3.0 | 3.0 | 75.1 | 1.5 | 64.4 | 0.9 | 73.6 | 63.5 |
| 24 | 2.5 | 8.0 | 8.0 | 76.3 | 2.4 | 74.6 | 0.8 | 73.9 | 73.8 |
| 26 | 2.0 | 6.0 | 2.0 | 63.4 | ND | 56.3 | 8.5 | 63.4 | 47.8 |
| 48 | 1.3 | 4.5 | 2.5 | 69.0 | 1.6 | 63.5 | 0.9 | 67.4 | 62.6 |
| 60 | 50.0 | 35.0 | 15.0 | 73.1 | 4.8 | 64.2 | 0.8 | 68.3 | 63.4 |
2.3.3. Immunoreactivity of cGMP 212Pb-TCMC-Trastuzumab
2.4. Effects of Storage at Higher Temperatures on cGMP TCMC-Trastuzumab

| Sample | Temp. (°C) | Time Point | Species | Analysis 1 | Analysis 2 | ||
|---|---|---|---|---|---|---|---|
| Retention (min) | % | Retention (min) | % | ||||
| Reference | 4 | 0 | HMW | 13.1 | 1.3 | 12.8 | 1.4 |
| IgG | 15.4 | 91.2 | 15.4 | 88.8 | |||
| LMW | 19.5 | 6.7 | 19.4 | 9.1 | |||
| cGMP | 4 | 0 | HMW | ||||
| IgG | 15.7 | 100 | 15.8 | 100 | |||
| LMW | |||||||
| cGMP | 21 | 2 weeks | HMW | ||||
| IgG | 15.8 | 100 | 15.8 | 100 | |||
| LMW | |||||||
| cGMP | 21 | 1 month | HMW | ||||
| IgG | 15.9 | 100 | 15.8 | 100 | |||
| LMW | |||||||
| cGMP | 21 | 3 months | HMW | ||||
| IgG | 16.0 | 100 | 15.9 | 100 | |||
| LMW | |||||||
| cGMP | 37 | 2 weeks | HMW | ||||
| IgG | 15.8 | 98.0 | 15.7 | 98.5 | |||
| LMW | 22.1 | 2.0 | 22.1 | 1.5 | |||
| cGMP | 37 | 1 month | HMW | ||||
| IgG | 15.8 | 97.7 | 15.7 | 98.5 | |||
| LMW | 22.1 | 2.3 | 22.1 | 1.5 | |||
| cGMP | 37 | 3 months | HMW | ||||
| IgG | 15.8 | 82.1 | 15.8 | 83.0 | |||
| LMW | 19.6 | 17.0 | 19.8 | 14.0 | |||
2.5. Effects of Storage at −80 °C as Well as Cycles of Freezing and Thawing on cGMP TCMC-Trastuzumab

3. Discussion
3.1. Requirements of Bringing a Radiolabeled Monoclonal Antibody to a Clinical Trial
3.2. Evaluation of A GMP mAb, TCMC-Trastuzumab, in Support of a Clinical Trial
3.3. Effects of Storage at Higher Temperatures on cGMP TCMC-Trastuzumab
3.4. Effects of Storage at −80 °C as Well as Cycles of Freezing and Thawing on cGMP TCMC-Trastuzumab
4. Experimental Section
4.1. Chelate Synthesis and mAb Conjugation
4.2. Arsenazo Assay
4.3. SDS-Polyacrylamide Gel Electrophoresis
4.4. Radiolabeling
4.4.1. Radio-Iodination with 125I
4.4.2. Radiolabeling with 212Pb
4.4.3. Radiolabeling with 203Pb
4.5. Instant Thin Layer Chromatography (ITLC)
4.6. Radioimmunoassays
4.6.1. Immunoreactivity of cGMP TCMC-Trastuzumab Assessed by Competition Radioimmunoassay
4.6.2. Immunoreactivity of Radiolabeled TCMC-Trastuzumab
4.7. High Performance Liquid Chromatography (HPLC)
4.7.1. Size-Exclusion (SE) HPLC
4.7.2. Ion-Exchange (IEX) HPLC
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflict of Interests
Abbreviations
| RIT | radioimmunotherapy |
| UAB | University of Alabama at Birmingham |
| TCMC | 1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamoylmethyl)-cyclododecane |
| cGMP | current good manufacturing practices |
| IEX-HPLC | ion-exchange high performance liquid chromatography |
| RIA | radioimmunoassay |
| SE-HPLC | Size-exclusion high performance liquid chromatography |
| mAb | monoclonal antibody |
| FDA | Food and Drug Administration |
| CRADA | Collaborative reasearch and development agreement |
| RICS | Radioimmune & Inorganic Chemistry Section |
| IND | Investigational new drug |
| IHC | immunohistochemistry |
| IC | immunoconjugate |
| BSA | bovine serum albumin |
| C/P | chelate-to-protein ratio |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| IgG | immunoglobulin |
| SOPs | standard operating protocols |
References
- US Food and Drug Administration. New Molecular Entity and New Therapeutic Biological Product Approvals for 2014. Available online: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm429247 (accessed on 24 July 2015).
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. MAbs 2015, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Abdulla, A.; Flynn, J.; Brechbiel, M.W. Potentiation of high-LET radiation by gemcitabine: Targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin. Cancer Res. 2007, 13, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2004, 10, 7834–7841. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother. Radiopharm. 2005, 20, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Baidoo, K.E.; Albert, P.S.; Wong, K.J.; Flynn, J.; Brechbiel, M.W. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2008, 14, 5108–5115. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Roselli, M.; Brechbiel, M.W.; Pippin, C.G.; McMurray, T.J.; Carrasquillo, J.A.; Colcher, D.; Lambrecht, R.; Gansow, O.A.; Schlom, J. In vivo evaluation of a lead-labeled monoclonal antibody using the DOTA ligand. Eur. J. Nucl. Med. 1998, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.G.; Schiffhauer, L. Standardized assessment of the HER2 status in breast cancer immunohistochemistry. Lab. Med. 2011, 42, 459–467. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Eppler, A.; Weigandt, M.; Schulze, S.; Hanefeld, A.; Bunjes, H. Comparison of different protein concentration techniques within preformulation development. Int. J. Pharm. 2011, 421, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Ren, D.; Xiao, G.; Bondarenko, P.; Sloey, C.; Ricci, M.S.; Krishnan, S. Elucidation of degradants in acidic peak of cation exchange chromatography in an IgG1 monoclonal antibody formed on long-term storage in a liquid formulation. Pharm. Res. 2012, 29, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Vieillard, V.; Da Silva Lemos, R.; Escalup, L.; Astier, A. Long-term physico-chemical stability of diluted trastuzumab. Int. J. Pharm. 2013, 448, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Goldspiel, J.T.; Goldspiel, B.R.; Grimes, G.J.; Yuan, P.; Potti, G. Stability of alemtuzumab solutions at room temperature. Am. J. Health Syst. Pharm. 2013, 70, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Ikesue, H.; Vermeulen, L.C.; Hoke, R.; Kolesar, J.M. Stability of cetuximab and panitumumab in glass vials and polyvinyl chloride bags. Am. J. Health Syst. Pharm. 2010, 67, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vermeulen, L.C.; Kolesar, J.M. Stability of stock and diluted rituximab. Am. J. Health Syst. Pharm. 2013, 70, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Kolesar, J.M.; Vermeulen, L. Assays for biological agents. Am. J. Health Syst. Pharm. 2013, 70, 1101. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Deckers, R.; Sasaki, N.; Bos, C.; Moonen, C. Mild hyperthermia influence on Herceptin® properties. Radiol. Oncol. 2015, 49, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Pabari, R.M.; Ryan, B.; McCarthy, C.; Ramtoola, Z. Effect of microencapsulation shear stress on the structural integrity and biological activity of a model monoclonal antibody, trastuzumab. Pharmaceutics 2011, 3, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Rathore, N.; Rajan, R.S. Current perspectives on stability of protein drug products during formulation, fill and finish operations. Biotechnol. Prog. 2008, 24, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Lazar, K.L.; Patapoff, T.W.; Sharma, V.K. Cold denaturation of monoclonal antibodies. MAbs 2010, 2, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef]
- Dadachova, E.; Chappell, L.L.; Brechbiel, M.W. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl. Med. Biol. 1999, 26, 977–982. [Google Scholar] [CrossRef]
- Fraker, P.J.; Speck, J.C., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Garmestani, K.; Milenic, D.E.; Brady, E.D.; Plascjak, P.S.; Brechbiel, M.W. Purification of cyclotron-produced 203Pb for labeling Herceptin. Nucl. Med. Biol. 2005, 32, 301–305. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Bench to Bedside: Stability Studies of GMP Produced Trastuzumab-TCMC in Support of a Clinical Trial. Pharmaceuticals 2015, 8, 435-454. https://doi.org/10.3390/ph8030435
Milenic DE, Baidoo KE, Brechbiel MW. Bench to Bedside: Stability Studies of GMP Produced Trastuzumab-TCMC in Support of a Clinical Trial. Pharmaceuticals. 2015; 8(3):435-454. https://doi.org/10.3390/ph8030435
Chicago/Turabian StyleMilenic, Diane E., Kwamena E. Baidoo, and Martin W. Brechbiel. 2015. "Bench to Bedside: Stability Studies of GMP Produced Trastuzumab-TCMC in Support of a Clinical Trial" Pharmaceuticals 8, no. 3: 435-454. https://doi.org/10.3390/ph8030435
APA StyleMilenic, D. E., Baidoo, K. E., & Brechbiel, M. W. (2015). Bench to Bedside: Stability Studies of GMP Produced Trastuzumab-TCMC in Support of a Clinical Trial. Pharmaceuticals, 8(3), 435-454. https://doi.org/10.3390/ph8030435