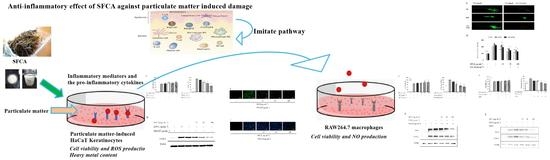
Anti-Adhesive Properties of Calcium Alginate from Sargassum fusiforme against Particulate Matter-Induced Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Calcium Alginate
2.3. Analysis of Composition and Heavy Metal Content
2.4. Cell Culture
2.5. Measurement of Cell Viability, ROS Production, and Nuclear Staining
2.6. Inflammatory Responses and Enzyme Immunoassay Measurement
2.7. Western Blot Assay
2.8. Zebrafish Embryo Assay
2.9. Statistical Analysis
3. Results
3.1. Chemical and Structural Features of SFCA
3.2. Compositional Analysis of PM-Stimulated Keratinocytes with/without SFCA Treatment
3.3. Protective Effect of SFCA against PM-Induced Inflammation in Keratinocytes
3.4. Protective Effect of SFCA against PM-Induced Apoptotic Body Formation in Keratinocytes
3.5. Protective Effect of SFCA in PM-Stimulated Macrophages
3.6. Inflammatory Responses in Macrophages Induced with H-PM and SFCA
3.7. Protective Effect of SFCA in the PM-Stimulated In Vivo Zebrafish Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dautrebande, L.; Beckmann, H.; Walkenhorst, W. Lung deposition of fine dust particles. Arch. Ind. Health 1957, 16, 179–187. [Google Scholar]
- Chan, C.K.; Yao, X. Air pollution in mega cities in China. Atmos. Environ. 2008, 42, 1–42. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, Z.; Wang, T.; Lian, H.; Sun, Y.; Wu, J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2. 5 in Nanjing, China. Atmos. Environ. 2012, 57, 146–152. [Google Scholar] [CrossRef]
- Charlesworth, S.; De Miguel, E.; Ordóñez, A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Smith, K.R. Household air pollution from coal and biomass fuels in China: Measurements, health impacts, and interventions. Environ. Health Perspect. 2007, 115, 848–855. [Google Scholar] [CrossRef]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Inflammatory mediators induced by coarse (PM2. 5–10) and fine (PM2. 5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 243–254. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, J. NKT-cell glycolipid agonist as adjuvant in synthetic vaccine. Carbohydr. Res. 2017, 452, 78–90. [Google Scholar] [CrossRef]
- Heck, D.E.; Gerecke, D.R.; Vetrano, A.M.; Laskin, J.D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. 2004, 195, 288–297. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Jiang, S.-J. Application of HPLC-ICP-MS and HPLC-ESI-MS procedures for arsenic speciation in seaweeds. J. Agric. Food Chem. 2012, 60, 2083–2089. [Google Scholar] [CrossRef]
- Soulairol, I.; Sanchez-Ballester, N.M.; Aubert, A.; Tarlier, N.; Bataille, B.; Quignard, F.; Sharkawi, T. Evaluation of the super disintegrant functionnalities of alginic acid and calcium alginate for the design of orodispersible mini tablets. Carbohydr. Polym. 2018, 197, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Cajnko, M.M.; Novak, U.; Likozar, B. Cascade valorization process of brown alga seaweed Laminaria hyperborea by isolation of polyphenols and alginate. J. Appl. Phycol. 2019, 31, 3915–3924. [Google Scholar] [CrossRef]
- Sarithakumari, C.; Renju, G.; Kurup, G.M. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 2013, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Jiang, Y.-F.; Lu, Y.-A.; Kang, M.-C.; Jeon, Y.-J. Fucoidan from acid-processed Hizikia fusiforme attenuates oxidative damage and regulate apoptosis. Int. J. Biol. Macromol. 2020, 160, 390–397. [Google Scholar] [CrossRef]
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.-J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.A.; Kim, S.-Y.; Lee, J.-S.; Jeon, Y.-J. Reduction of heavy metal (Pb2+) biosorption in zebrafish model using alginic acid purified from Ecklonia cava and two of its synthetic derivatives. Int. J. Biol. Macromol. 2018, 106, 330–337. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Grasdalen, H. High-field, 1H-n.m.r. spectroscopy of alginate: Sequential structure and linkage conformations. Carbohydr. Res. 1983, 118, 255–260. [Google Scholar] [CrossRef]
- Fernando, I.; Kim, H.-S.; Sanjeewa, K.; Oh, J.-Y.; Jeon, Y.-J.; Lee, W.W. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Kang, S.-M.; Sok, C.H.; Hong, J.T.; Oh, J.-Y.; Jeon, Y.-J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163. [Google Scholar]
- Dai, Y.-L.; Jiang, Y.-F.; Lu, Y.-A.; Yu, J.-B.; Kang, M.-C.; Jeon, Y.-J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Ma, C.; Xing, S.; Sun, L.; Huang, L. A review of fundamental factors affecting diesel PM oxidation behaviors. Sci. China Technol. Sci. 2018, 61, 330–345. [Google Scholar] [CrossRef]
- Wang, H.; Song, Q.; Yao, Q.; Chen, C.H. Experimental study on removal effect of wet flue gas desulfurization system on fine particles from a coal-fired power plant. Proc. Chin. Soc. Electr. Eng. 2008, 28, 1–7. [Google Scholar]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2. 5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Wang, Y.; Li, W.; Zhang, Q.; Wang, W.; Quan, J.; Cao, G.; Wang, J.; Yang, Y. Factors contributing to haze and fog in China. Chin. Sci. Bull. 2013, 58, 1178–1187. [Google Scholar]
- Sheng, L.T.; Fei, G.X.; Sheng, A.Z.; Xiang, F.Y. The dust fall in Beijing, China on 18 April 1980. In Desert Dust: Origin, Characteristics, and Effect on Man; Geological Society of America: Boulder, CO, USA, 1981; Volume 186, p. 149. [Google Scholar]
- Zeng, X.; Xu, X.; Zheng, X.; Reponen, T.; Chen, A.; Huo, X. Heavy metals in PM2. 5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ. Pollut. 2016, 210, 346–353. [Google Scholar] [CrossRef]
- Gross, J.E.; Carlos, W.G.; Dela Cruz, C.S.; Harber, P.; Jamil, S. Sand and dust storms: Acute exposure and threats to respiratory health. Am. J. Respir. Crit. Care Med. 2018, 198, P13–P14. [Google Scholar] [CrossRef]
- Kim, I. Toxic compounds analysis and risk assessment for fine dust (PM2. 5) in Gwangju. Symp. Conf. Soc. Environ. Toxic. Health 2018, 6, 139–153. [Google Scholar]
- Schripp, T.; Braun, M.; Grein, T.; Oßwald, P. Ambient Ultra-Fine Particle Exposure Assessment Based on Mobile and Stationary Monitoring during Fine Dust Season in Stuttgart, Germany. In Proceedings of the ISEE Conference Abstracts, Ottawa, ON, Canada, 26–30 August 2018. [Google Scholar]
- Wang, H.; Qiao, L.; Lou, S.; Zhou, M.; Ding, A.; Huang, H.; Chen, J.; Wang, Q.; Tao, S.; Chen, C. Chemical composition of PM2. 5 and meteorological impact among three years in urban Shanghai, China. J. Clean. Prod. 2016, 112, 1302–1311. [Google Scholar] [CrossRef]
- Xu, H.; Cao, J.; Chow, J.C.; Huang, R.-J.; Shen, Z.; Chen, L.A.; Ho, K.F.; Watson, J.G. Inter-annual variability of wintertime PM2. 5 chemical composition in Xi’an, China: Evidences of changing source emissions. Sci. Total Environ. 2016, 545, 546–555. [Google Scholar] [CrossRef]
- Chen, P.; Bi, X.; Zhang, J.; Wu, J.; Feng, Y. Assessment of heavy metal pollution characteristics and human health risk of exposure to ambient PM2. 5 in Tianjin, China. Particuology 2015, 20, 104–109. [Google Scholar] [CrossRef]
- Bekki, K.; Ito, T.; Yoshida, Y.; He, C.; Arashidani, K.; He, M.; Sun, G.; Zeng, Y.; Sone, H.; Kunugita, N. PM2. 5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ. Toxicol. Pharmacol. 2016, 45, 362–369. [Google Scholar] [CrossRef]
- Jin, X.-T.; Chen, M.-L.; Li, R.-J.; An, Q.; Song, L.; Zhao, Y.; Xiao, H.; Cheng, L.; Li, Z.-Y. Progression and inflammation of human myeloid leukemia induced by ambient PM2. 5 exposure. Arch. Toxicol. 2016, 90, 1929–1938. [Google Scholar] [CrossRef]
- Hu, R.; Xie, X.-Y.; Xu, S.-K.; Wang, Y.-N.; Jiang, M.; Wen, L.-R.; Lai, W.; Guan, L. PM2. 5 exposure elicits oxidative stress responses and mitochondrial apoptosis pathway activation in HaCaT keratinocytes. Chin. Med. J. 2017, 130, 2205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Tuo, J.; Liu, Q.; Zhang, X.; Xu, Z.; Liu, S.; Sui, G. Analysis of PM2. 5-induced cytotoxicity in human HaCaT cells based on a microfluidic system. Toxicol. Vitr. 2017, 43, 1–8. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 2010, 10, 365. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Sanjeewa, K.A.; Fernando, I.S.; Ryu, B.M.; Kang, M.-C.; Jee, Y.; Lee, W.W.; Jeon, Y.-J. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement. Altern. Med. 2018, 18, 249. [Google Scholar] [CrossRef] [Green Version]
- Calumpong, H.; Maypa, A.; Magbanua, M. Population and alginate yield and quality assessment of four Sargassum species in Negros Island, central Philippines. Hydrobiologia 1999, 398, 211–215. [Google Scholar] [CrossRef]
- Schaumann, K.; Weide, G. Enzymatic degradation of alginate by marine fungi. In Proceedings of the Thirteenth International Seaweed Symposium, Vancouver, BC, Canada, 13–18 August 1989; pp. 589–596. [Google Scholar]
- Haug, A.; Larsen, B.; Smidsrød, O. Uronic acid sequence in alginate from different sources. Carbohydr. Res. 1974, 32, 217–225. [Google Scholar] [CrossRef]







| Constituent | Composition |
|---|---|
| Carbohydrate | 89.25 ± 0.64% |
| Ash | 2.41 ± 0.27% |
| Polyphenol | 0.96 ± 0.01% |
| Protein | 0.41 ± 0.02% |
| M/G ratio | 1.25 |
| Element (ppm) | Control | PM | PM+SFCA | PM+SFCA | PM+SFCA |
|---|---|---|---|---|---|
| (25 μg mL−1) | (50 μg mL−1) | (100 μg mL−1) | |||
| K | 454 ± 32 | 451 ± 32 | 456 ± 24 | 435 ± 26 | 424 ± 38 |
| Ca | 149 ± 15 * | 435 ± 21 | 345 ± 26 | 159 ± 38 | 62 ± 25 ** |
| Na | 612 ± 9 | 609 ± 29 | 623 ± 22 | 657 ± 34 | 665 ± 25 |
| Mg | 115 ± 9 | 165 ± 23 | 132 ± 21 | 65 ± 14 | 38 ± 10 ** |
| Sr | 5 ± 6 ** | 149 ± 19 | 94 ± 12 * | 44 ± 18 * | 18 ± 20 ** |
| Fe | 49 ± 9 ** | 258 ± 35 | 165 ± 11 | 140 ± 25 * | 94 ± 33 ** |
| Al | N.D. | 79 ± 14 | 75 ± 4 | 62 ± 8 | 51 ± 14 |
| As | N.D. | 39 ± 16 | 42 ± 18 | 31 ± 11 | 19 ± 6 |
| Mn | N.D. | 65 ± 6 | 45 ± 15 | 32 ± 14 | 11 ± 9 |
| Pb | N.D. | 368 ± 15 | 217 ± 38 | 111 ± 15 * | 39 ± 25 ** |
| Cu | N.D. | 32 ± 15 | 32 ± 7 | 11 ± 10 | 1 ± 3 |
| Cr | N.D. | 32 ± 11 | 25 ± 15 | 12 ± 24 | N.D. |
| Ba | N.D. | 101 ± 11 | 78 ± 28 | 14 ± 12 | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.-H.; Tao, X.-Y.; Yang, D.; Li, X.; Zhou, D.-Y.; Dai, Y.-L.; Jeon, Y.-J. Anti-Adhesive Properties of Calcium Alginate from Sargassum fusiforme against Particulate Matter-Induced Inflammation. Curr. Issues Mol. Biol. 2022, 44, 626-639. https://doi.org/10.3390/cimb44020043
Fu Y-H, Tao X-Y, Yang D, Li X, Zhou D-Y, Dai Y-L, Jeon Y-J. Anti-Adhesive Properties of Calcium Alginate from Sargassum fusiforme against Particulate Matter-Induced Inflammation. Current Issues in Molecular Biology. 2022; 44(2):626-639. https://doi.org/10.3390/cimb44020043
Chicago/Turabian StyleFu, Yun-Hua, Xing-Yu Tao, Di Yang, Xue Li, Dong-Yue Zhou, Yu-Lin Dai, and You-Jin Jeon. 2022. "Anti-Adhesive Properties of Calcium Alginate from Sargassum fusiforme against Particulate Matter-Induced Inflammation" Current Issues in Molecular Biology 44, no. 2: 626-639. https://doi.org/10.3390/cimb44020043
APA StyleFu, Y. -H., Tao, X. -Y., Yang, D., Li, X., Zhou, D. -Y., Dai, Y. -L., & Jeon, Y. -J. (2022). Anti-Adhesive Properties of Calcium Alginate from Sargassum fusiforme against Particulate Matter-Induced Inflammation. Current Issues in Molecular Biology, 44(2), 626-639. https://doi.org/10.3390/cimb44020043