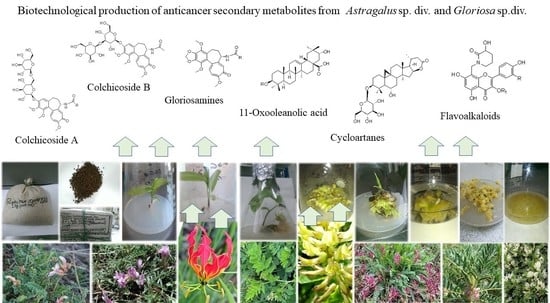
Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L.
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characteristics of Target Astragalus Species
3.2. Ethnobotanical Data of Astragalus Species Used against Cancer
3.3. Secondary Metabolites of Astragalus Species Anticancer Properties
| Plant Species | Type | Compounds Isolated | Cytotoxicity on Cell Lines (IC50) | References |
|---|---|---|---|---|
| A. aitosensis | callus, suspension | cycloartane saponins, sterols, flavonoids | n.d. | [51] |
| aerial pars, wild grown * | 5,6-dehydro-6-desoxyastragenol | n.d. | [51] | |
| A. angustifolius | callus, suspension | cycloartane saponins, flavonoids | n.d. | [53] |
| aerial parts, wild grown * | β-sitosterol, cycloastragenol, astragenol, soyasapogenol B, 3-O-[α-L-rha-(1→2)-β-D-xyl-(1→2)-β-D-glc]-3β,22β,24-trihydroxyolean-12-en-29-oic acid | n. d. HeLa (36 µM); HT-29 (50 µM) | [63] [53] | |
| A. asper | aerial parts, wild grown * | saponins, flavonoids | n.d. | [53] |
| A. boeticus | callus, suspension, hairy roots | saponins, soyasapogenol B, β-sitosterol, flavonoids | n.d. | [70] |
| A. brachycera | hairy roots ** shoots ** | cycloartane saponins, sterols | n.d. | [51] |
| A. canadensis | hairy roots | cycloartane saponins, cycloastragenol, astragenol, | n.d. | [51] |
| A. centralpinus | aerial parts, wild grown * | flavonoids | n.d. | [53] |
| A. corniculatus | aerial parts, wild grown *** | two oleanane type saponins and a corresponding lactone | Graffi tumour–in vivo, i.p., hamsters (50 mg/kg) ***; in vitro (20 µg/mL) *** | [71] |
| A. edulis | callus | quercetin, kaempferol, isorhamnetin, saponins | n.d. | [70] |
| A. englerianus | hairy roots | cycloartane saponins | n.d. | [51] |
| A. falcatus | hairy roots | cycloartane saponins | n.d. | [51] |
| A. glycypyllos | hairy roots **, callus shoots *** | cycloastragenol, astragenol, soyasapogenol B epoxycycloartanes | n.d. T-24 (125 µg/mL); CAL-29 (90 µg/mL); MJ (75 µg/mL); HUT-78 (78 µg/mL) | [51] [53] [72] |
| aerial parts, wild grown *** | epoxycycloartanes | K-562 (50 µg/mL) ***; HL-60 (40 µg/mL) ***; BV-173 (70 µg/mL) *** | [73] | |
| aerial parts, wild grown *** | epoxycycloartanes | T-24 (168 µg/mL); CAL-29 (105 µg/mL); MJ (126 µg/mL); HUT-78 (87 µg/mL) | [72] | |
| aerial parts, wild grown | 17(R),20(R)-3β,6α,16β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-O-β-D-glucopyranoside | T-24 (66 µg/mL); CAL-29 (52 µg/mL); MJ (52 µg/mL); HUT-78 (18 µg/mL) | [74] | |
| A. hamosus | callus, suspension, hairy roots | saponins, soyasapogenol B, β-sitosterol, astragalin, rutin, isorhamnetin-3-O-glycoside | n.d. | [53,70] |
| aerial parts, wild grown *** | saponins | HL-60 (63 µg/mL); HL-60/Dox (25 µg/mL); SKW-3 (84 µg/mL) | [75] | |
| A. missouriensis | Callus **, suspension, hairy roots | isoquercitrin, quercitrin, rutin, hyperoside, saponins | n.d. | [70,71] |
| A. mongholicus (syn. A. membranaceus) | hairy roots **, shoots ** | astragalosides, β-sitosterol, stigmasterol, campesterol | n.d. | [51,72] |
| A. monspessulanus | aerial parts, wild grown * | flavoalkaloids, acylated flavonoids, flavonoids | n.d. | [65] |
| A. onobrychis | aerial parts, wild grown * | flavonoids, saponins | n.d. | [76] |
| A. oxyglotis | hairy roots | cycloartane saponins | n.d. | [51] |
| A. sesameus | Shoots ** | - | HL-60/Dox (87 µg/mL); SKW-3 (68 µg/mL) | |
| A. spruneri | aerial parts, wild grown * | flavonoids | n.d. | [77] |
| A. sulcatus | hairy roots | cycloartane saponins, sterols, swensonine | n.d. | [51] |
| A. thracicus | callus, suspension | saponins, flavonoids | n.d. | [53] |
| aerial parts, wild grown * | saponins, flavonoids | HT-29 (52 µg/mL); HL-60 (67 µg/mL); HL-60/Dox (53 µg/mL); SKW-3 (83 µg/mL) | [53] | |
| A. vesicarius ssp. carniolicus | callus | flavonoids | HL-60 (8.8 µg/mL) *; HL-60/Dox (11.8 µg/mL) * | [78] |
| callus | 5-hydroxy-7-methoxy-2′, 5′-dihydroxyisoflavone | HL-60 (38.9 µg/mL); HL-60/Dox (35.2 µg/mL) | [78] | |
| 5, 7-dihydroxy-4′-methoxyisoflavone | HL-60 (41.4 µg/mL); HL-60/Dox (42.4 µg/mL) | [78] | ||
| 7-methoxy-5-hydroxy-4′-methoxy-2′-hydroxyisoflavone | HL-60 (64.1 µg/mL); HL-60/Dox (41.8 µg/mL) | [78] | ||
| 8-pregnyl genistein | HL-60 (36.1 µg/mL); HL-60/Dox (36.1 µg/mL) | [78] | ||
| 5,7-dihydroxy-8-pregnyl-4′-methoxy-2′-hydroxyisoflavone | HL-60 (56.3 µg/mL); HL-60/Dox (56.8 µg/mL) | [78] | ||
| sophorophenolone | HL-60 (78.0 µg/mL); HL-60/Dox (63.0 µg/mL) | [78] | ||
| G. superba | seeds | colchicoside, colchicine, 3-O-demethylcolchicine | PANC-1, PANC02 (GS ++ 0.45–0.59 µg/mL) PANC02 (GS2B + 9.49 µg/mL) | [79] [80] |
| glorioside, colchicodiside A, gloriodiside, colchicodiside B, colchicodiside C, dongduengoside A-C, colchicine, 2-demethilcolchicine, colchicoside and luteolin 7-O-β-D-glucopyranoside | DLA (29 µg #; 21 µg ##) | [81] [82] [83] | ||
| rhizomes | peptides | SW620 (n.d.) | [84] | |
| roots | colchicine | HT-29 (0.12 μg/mL *) | [85] | |
| G. rothschildiana | aerial parts | gloriosamine A-D, colchicine, colchiciline, colchifoline and N-deacetyl-N-formylcolchicine | - | [86] |
3.4. Biotechnology of Astragalus Species
3.4.1. Cell Culture
3.4.2. Effects of Medium Composition and Growth Regulators
3.4.3. Effects of End-Product Inhibition
3.4.4. Genetic Transformation of Astragalus Species by Agrobacterium Rhizogenes
3.4.5. In Vitro Production of Secondary Metabolite
3.5. Characteristics of Target Gloriosa Species
3.5.1. Ethnobotaical Data of Gloriosa Species Used against Cancer
3.5.2. Secondary Metabolites of Gloriosa Species with Anticancer Properties
3.5.3. Biotechnology of Gloriosa Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Understanding Cancer Causes: Ancient Times to Present; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Clark, A.M. Natural Products as a Resource for New Drugs. Pharm. Res. 1996, 13, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Alfermann, A.W.; Franke, R.; Wetterauer, B.; Distl, M.; Windhövel, J.; Krohn, O.; Fuss, E.; Garden, H.; Mohagheghzadeh, A.; et al. Sustainable Bioproduction of Phytochemicals by Plant in Vitro Cultures: Anticancer Agents. Plant Genet. Resour. 2005, 3, 90–100. [Google Scholar] [CrossRef]
- DiGiulio, S. 3 QUESTIONS ON… Cancer Rates in Medieval Populations: With Piers Mitchell, MD, Director of the Ancient Parasites Laboratory at University of Cambridge. Oncol. Times 2021, 43, 42. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Dittmar, J.M.; Mulder, B.; Inskip, S.; Littlewood, A.; Cessford, C.; Robb, J.E. The Prevalence of Cancer in Britain before Industrialization. Cancer 2021, 127, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Senga, S.S.; Grose, R.P. Hallmarks of Cancer—the New Testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food Applications of Natural Antimicrobial Compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef]
- Greger, H. Phytocarbazoles: Alkaloids with Great Structural Diversity and Pronounced Biological Activities. Phytochem. Rev. 2017, 16, 1095–1153. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The Evolving Role of Natural Products in Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Akerele, O.; Heywood, V.; Synge, H. (Eds.) Conservation of Medicinal Plants, 1st ed.; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar] [CrossRef]
- Hamilton, A.C. Medicinal Plants, Conservation and Livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Evstatieva, L.; Hardalova, R.; Stoyanova, K. Medicinal Plants in Bulgaria: Diversity, Legislation, Conservation and Trade. Phytol. Balc. 2007, 13, 415–427. [Google Scholar]
- Soetan, K.O.; Aiyelaagbe, O.O. The Need for Bioactivity-Safety Evaluation and Conservation of Medicinal Plants—A Review. J. Med. Plants Res. 2009, 3, 324–328. [Google Scholar]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Schippmann, U.; Leaman, D.; Cunningham, A. Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. In Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries; United Nations: San Francisco, CA, USA, 2002; pp. 142–167. [Google Scholar]
- Ramawat, K.G.; Arora, J. Medicinal Plants Domestication, Cultivation, Improvement, and Alternative Technologies for the Production of High Value Therapeutics: An Overview. In Medicinal Plants; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2021; Volume 28, pp. 1–29. [Google Scholar] [CrossRef]
- Cao, P.; Wang, G.; Wei, X.; Chen, S.; Han, J. How to Improve CHMs Quality: Enlighten from CHMs Ecological Cultivation. Chin. Herb. Med. 2021, 13, 301–312. [Google Scholar] [CrossRef]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing Medicinal Plants into Cultivation: Opportunities and Challenges for Biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Mora-Vásquez, S.; Wells-Abascal, G.G.; Espinosa-Leal, C.; Cardineau, G.A.; García-Lara, S. Application of Metabolic Engineering to Enhance the Content of Alkaloids in Medicinal Plants. Metab. Eng. Commun. 2022, 14, e00194. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary Metabolism of Pharmaceuticals in the Plant in Vitro Cultures: Strategies, Approaches, and Limitations to Achieving Higher Yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically Active Natural Product Synthesis and Supply via Plant Cell Culture Technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Pavlov, A.; Popov, S.; Kovacheva, E.; Georgiev, M.; Ilieva, M. Volatile and Polar Compounds in Rosa Damascena Mill 1803 Cell Suspension. J. Biotechnol. 2005, 118, 89–97. [Google Scholar] [CrossRef]
- Frodin, D.G. History and Concepts of Big Plant Genera. Taxon 2004, 53, 753–776. [Google Scholar] [CrossRef]
- Podlech, D. Taxonomic and Phytogeographical Problems in Astragalus of the Old World and South-West Asia. Proc. Sect. B Biol. Sci. 1986, 89, 37–43. [Google Scholar] [CrossRef]
- IUCN. Available online: https://www.iucnredlist.org/search?query=astragalus&searchType=species (accessed on 1 January 2022).
- Xu, L.; Podlech, D. Astragalus Mongholicus Bunge. Flora China 2010, 10, 338–339, 343. [Google Scholar]
- Tierra, M.; Tierra, L. Chinese Traditional Herbal Medicine; Lotus Light Pub: Twin Lakes, WI, USA, 1998. [Google Scholar]
- Shahrajabian, M.H. A Review of Astragalus Species as Foodstuffs, Dietary Supplements, a Traditional Chinese Medicine and A Part of Modern Pharmaceutical Science. Appl. Ecol. Env. Res. 2019, 17, 13371–13382. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, Q.; Jiao, L.J.; Huang, Y.L.; Garfield, D.; Zhang, J.; Xu, L. Astragalus-Containing Traditional Chinese Medicine, with and without Prescription Based on Syndrome Differentiation, Combined with Chemotherapy for Advanced Non-Small-Cell Lung Cancer: A Systemic Review and Meta-Analysis. Curr. Oncol. 2016, 23, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.S.; Joharchi, M.R.; Nadaf, M.; Nasseh, Y. Ethnobotanical Knowledge of Astragalus spp.: The World’s Largest Genus of Vascular Plants. Avicenna J. Phytomed. 2020, 10, 128–142. [Google Scholar]
- Ahtarov, B.; Davidov, B.; Yavashev, A. Materials for the Bulgarian Botanical Glossary; Balgarska Akademia na Naukite Pridvorna Pechatnitsa: Sofia, Bulgaria, 1939. [Google Scholar]
- Guarino, C. Ethnobotanical Study of the Sannio Area, Campania, Southern Italy. Ethnobot. Res. App. 2008, 6, 255. [Google Scholar] [CrossRef]
- Sezik, E.; Yeşilada, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional Medicine in Turkey X. Folk Medicine in Central Anatolia. J. Ethnopharmacol. 2001, 75, 95–115. [Google Scholar] [CrossRef]
- Cakilcioglu, U.; Turkoglu, I. An Ethnobotanical Survey of Medicinal Plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Tetik, F.; Civelek, S.; Cakilcioglu, U. Traditional Uses of Some Medicinal Plants in Malatya (Turkey). J. Ethnopharmacol. 2013, 146, 331–346. [Google Scholar] [CrossRef]
- Mükemre, M.; Behçet, L.; Çakılcıoğlu, U. Ethnobotanical Study on Medicinal Plants in Villages of Çatak (Van-Turkey). J. Ethnopharmacol. 2015, 166, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Nadiroğlu, M.; Behçet, L.; Çakılcıoğlu, U. An Ethnobotanical Survey of Medicinal Plants in Karlıova (Bingöl-Turkey). Indian J. Tradit. Knowl. 2019, 18, 76–87. [Google Scholar]
- Ergül Bozkurt, A.E. Folk Medicinal Plants Used for Treatment of Gynecological Disorders by Rural Population of Zorlu Village (in Turkey). Ethnobot. Res. App. 2021, 22, 1–17. [Google Scholar] [CrossRef]
- Bozyel, M.E.; Merdamert Bozyel, E.; Canli, K.; Altuner, E.M. Türk Geleneksel Tıbbında Tıbbi Bitkilerin Antikanser Kullanımları. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg. 2019, 22. [Google Scholar] [CrossRef]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2019. [Google Scholar]
- Bussmann, R.W.; Zambrana, P.; Narel, Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Hart; Robbie, E. Ethnobotany of Samtskhe-Javakheti, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017, 16, 7–24. [Google Scholar]
- Lysiuk, R.; Darmohray, R. Pharmacology and Ethnomedicine of the Genus Astragalus. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 3, 46–53. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus Membranaceus: A Review of Its Protection Against Inflammation and Gastrointestinal Cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- McCulloch, M.; See, C.; Shu, X.; Broffman, M.; Kramer, A.; Fan, W.; Gao, J.; Lieb, W.; Shieh, K.; Colford, J.M. Astragalus-Based Chinese Herbs and Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer: Meta-Analysis of Randomized Trials. JCO 2006, 24, 419–430. [Google Scholar] [CrossRef]
- Lin, S.; An, X.; Guo, Y.; Gu, J.; Xie, T.; Wu, Q.; Sui, X. Meta-Analysis of Astragalus-Containing Traditional Chinese Medicine Combined With Chemotherapy for Colorectal Cancer: Efficacy and Safety to Tumor Response. Front. Oncol. 2019, 9, 749. [Google Scholar] [CrossRef]
- Ali, M.; Aldosari, A.; Tng, D.Y.P.; Ullah, M.; Hussain, W.; Ahmad, M.; Hussain, J.; Khan, A.; Hussain, H.; Sher, H.; et al. Traditional Uses of Plants by Indigenous Communities for Veterinary Practices at Kurram District, Pakistan. Ethnobot. Res. Appl. 2019, 18, 1–19. [Google Scholar] [CrossRef]
- Cho, W.C. Immunomodulatory and Anti-Tumor Activities of Astragalus Ancient Herb—Modern Miracle; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2009. [Google Scholar]
- Li, R.; Chen, W.; Wang, W.; Tian, W.; Zhang, X. Extraction, Characterization of Astragalus Polysaccharides and Its Immune Modulating Activities in Rats with Gastric Cancer. Carbohydr. Polym. 2009, 78, 738–742. [Google Scholar] [CrossRef]
- Ionkova, I.; Shkondrov, A.; Krasteva, I.; Ionkov, T. Recent Progress in Phytochemistry, Pharmacology and Biotechnology of Astragalus Saponins. Phytochem. Rev. 2014, 13, 343–374. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A Review of Recent Research Progress on the Astragalus Genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.; Shkondrov, A.; Ionkova, I.; Zdraveva, P. Advances in Phytochemistry, Pharmacology and Biotechnology of Bulgarian Astragalus Species. Phytochem. Rev. 2016, 15, 567–590. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus Membranaceus Inhibit Breast Cancer Cells Proliferation via PI3K/AKT/MTOR Signaling Pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Wang, J.; Ren, B.; Zhang, L.; Li, W. Formononetin, an Isoflavone from Astragalus Membranaceus Inhibits Proliferation and Metastasis of Ovarian Cancer Cells. J. Ethnopharmacol. 2018, 221, 91–99. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-Tumor Potential of Astragalus Polysaccharides on Breast Cancer Cell Line Mediated by Macrophage Activation. Mater. Sci. Eng. C 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; Guo, Y.; Wang, H.; et al. Anti-Tumor Effects and Mechanisms of Astragalus Membranaceus (AM) and Its Specific Immunopotentiation: Status and Prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef]
- Shen, L.; Gwak, S.R.; Cui, Z.Y.; Joo, J.C.; Park, S.J. Astragalus-Containing Chinese Herbal Medicine Combined With Chemotherapy for Cervical Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 587021. [Google Scholar] [CrossRef]
- Le Marchand, L. Cancer Preventive Effects of Flavonoids—A Review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary Polyphenolic Phytochemicals—Promising Cancer Chemopreventive Agents in Humans? A Review of Their Clinical Properties. Int. J. Cancer 2007, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zong, A.; Cao, H.; Wang, F. Anticancer Polysaccharides from Natural Resources: A Review of Recent Research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Lv, D.; Zhang, S.; Wang, Z.; Zhou, B. Astragaloside IV Inhibits the Progression of Non-Small Cell Lung Cancer Through the Akt/GSK-3β/β-Catenin Pathway. Ooncol. Res. 2019, 27, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Gülcemal, D.; Masullo, M.; Bedir, E.; Festa, M.; Karayıldırım, T.; Alankus-Caliskan, O.; Piacente, S. Triterpene Glycosides from Astragalus Angustifolius. Planta Med. 2012, 78, 720–729. [Google Scholar] [CrossRef]
- Bourezzane, S.; Haba, H.; Long, C.; Benkhaled, M. Chemical Composition and Antioxidant Activity of Astragalus Monspessulanus L. Growing in Semiarid Areas of Algeria. J. Serb. Chem. Soc. 2018, 83, 31–38. [Google Scholar] [CrossRef]
- Krasteva, I.; Bratkov, V.; Bucar, F.; Kunert, O.; Kollroser, M.; Kondeva-Burdina, M.; Ionkova, I. Flavoalkaloids and Flavonoids from Astragalus Monspessulanus. J. Nat. Prod. 2015, 78, 2565–2571. [Google Scholar] [CrossRef]
- Elenga, P.; Nikolov, S.; Panova, D. Triterpene Glycosides and Sterols from Astragalus Glycyphyllos L. Pharmazie 1986, 41, 300. [Google Scholar]
- Elenga, P.; Nikolov, S.; Panova, D. Triterpene Glycosides from Astragalus Glycyphyllos L.—A New Natural Compound of the Overground Parts. Pharmazie 1987, 42, 422–423. [Google Scholar]
- Linnek, J.; Mitaine-Offer, A.; Miyamoto, T.; Lacaille-Dubois, M. Two Cycloartane-Type Glycosides from the Roots of Astragalus Glycyphyllos. Planta Med. 2008, 74, PB141. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. A New Tetracyclic Saponin from Astragalus Glycyphyllos L. and Its Neuroprotective and HMAO-B Inhibiting Activity. Nat. Prod. Res. 2020, 34, 511–517. [Google Scholar] [CrossRef]
- Ionkova, I.; Alfermann, A. Transformation of Astragalus Species by Agrobacterium Rhizogenes and Their Saponin Production. Planta Med. 1990, 56, 634–635. [Google Scholar] [CrossRef]
- Georgieva, A.; Popov, G.; Shkondrov, A.; Toshkova, R.; Krasteva, I.; Kondeva-Burdina, M.; Manov, V. Antiproliferative and Antitumour Activity of Saponins from Astragalus Glycyphyllos on Myeloid Graffi Tumour. J. Ethnopharmacol. 2021, 267, 113519. [Google Scholar] [CrossRef] [PubMed]
- Shkondrov, A.; Krasteva, I.; Ionkova, I.; Popova, P.; Zarev, Y.; Mihaylova, R.; Konstantinov, S. Production of Saponins from in Vitro Cultures of Astragalus Glycyphyllos and Their Antineoplastic Activity. Biotechnol. Biotechnol. Equip. 2019, 33, 1413–1418. [Google Scholar] [CrossRef]
- Shkondrov, A. Phytochemical Investigation of Species from Genus Astragalus L. (Fabaceae); Medical University of Sofia: Sofia, Bulgaria, 2017. [Google Scholar]
- Mihaylova, R.; Shkondrov, A.; Aluani, D.; Ionkova, I.; Tzankova, V.; Krasteva, I. In Vitro Antitumour and Immunomodulating Activity of Saponins from Astragalus Glycyphyllos. Biotechnol. Biotechnol. Equip. 2021, 35, 1948–1955. [Google Scholar] [CrossRef]
- Krasteva, I.; Momekov, G.; Zdraveva, P.; Konstantinov, S.; Nikolov, S. Antiproliferative Effects of a Flavonoid and Saponins from Astragalus Hamosus against Human Tumor Cell Lines. Pharmacogn. Mag. 2008, 4, 269–272. [Google Scholar]
- Benbassat, N.; Nikolov, S. Flavonoids from Astragalus Onobrychis. Planta Med. 1995, 61, 100. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Shkondrov, A.; Simeonova, R.; Vitcheva, V.; Krasteva, I.; Ionkova, I. In Vitro/in Vivo Antioxidant and Hepatoprotective Potential of Defatted Extract and Flavonoids Isolated from Astragalus Spruneri Boiss. (Fabaceae). Food Chem. Toxicol. 2018, 111, 631–640. [Google Scholar] [CrossRef]
- Popova, P.; Zarev, Y.; Mihaylova, R.; Momekov, G.; Ionkova, I. Antiproliferative Activity of Extract from in Vitro Callus Cultures of Astragalus Vesicarius Ssp. Carniolicus (A. Kern.) Chater. Pharmacia 2021, 68, 217–221. [Google Scholar] [CrossRef]
- Capistrano, R.; Vangestel, C.; Wouters, A.; Dockx, Y.; Pauwels, P.; Stroobants, S.; Apers, S.; Lardon, F.; Pieters, L.; Staelens, S. Efficacy Screening of Gloriosa Superba Extracts in a Murine Pancreatic Cancer Model Using 18F-FDG PET/CT for Monitoring Treatment Response. Cancer Biother. Radiopharm. 2016, 31, 99–109. [Google Scholar] [CrossRef]
- Capistrano, I.R.; Vangestel, C.; Vanpachtenbeke, H.; Fransen, E.; Staelens, S.; Apers, S.; Pieters, L. Coadministration of a Gloriosa Superba Extract Improves the in Vivo Antitumoural Activity of Gemcitabine in a Murine Pancreatic Tumour Model. Phytomedicine 2016, 23, 1434–1440. [Google Scholar] [CrossRef]
- Zarev, Y.; Foubert, K.; Ionkova, I.; Apers, S.; Pieters, L. Isolation and Structure Elucidation of Glucosylated Colchicinoids from the Seeds of Gloriosa Superba by LC-DAD-SPE-NMR. J. Nat. Prod. 2017, 80, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Sahakitpichan, P.; Chimnoi, N.; Namsa-aid, A.; Panyadee, A.; Ruchirawat, S.; Kanchanapoom, T. Colchicinoid Glucosides from Seedless Pods of Thai Origin Gloriosa Superba. Phytochem. Lett. 2016, 16, 299–302. [Google Scholar] [CrossRef]
- Saradhadevi, M.; Gnanadesigan, M.; Kapildev, G.; Vasanth, D. Dataset on Antitumor Properties of Silver Nanoparticles from Gloriosa Superba (L.) Seed on Dalton Lymphoma Ascites (DLA) Tumor: Facile and Biocompatible Approach. Data Brief 2017, 14, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Budchart, P.; Khamwut, A.; Sinthuvanich, C.; Ratanapo, S.; Poovorawan, Y.; T-Thienprasert, N.P. Partially Purified Gloriosa Superba Peptides Inhibit Colon Cancer Cell Viability by Inducing Apoptosis Through P53 Upregulation. Am. J. Med. Sci. 2017, 354, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Akazawa, H.; Akihisa, T.; Jantrawut, P.; Kitdamrongtham, W.; Manosroi, W.; Manosroi, J. In Vitro Anti-Proliferative Activity on Colon Cancer Cell Line (HT-29) of Thai Medicinal Plants Selected from Thai/Lanna Medicinal Plant Recipe Database “MANOSROI III. ” J. Ethnopharmacol. 2015, 161, 11–17. [Google Scholar] [CrossRef]
- Kitajima, M.; Tanaka, A.; Kogure, N.; Takayama, H. Four New Colchicinoids, Gloriosamines A–D, from Gloriosa Rothschildiana. Tetrahedron Lett. 2008, 49, 257–260. [Google Scholar] [CrossRef]
- Krasteva, I.; Nikolov, S.; Kaloga, M.; Mayer, G. Triterpenoid Saponins from Astragalus Corniculatus. Z. Für. Nat. B 2006, 61, 1166–1169. [Google Scholar] [CrossRef]
- Krasteva, I.; Nikolov, S.; Kaloga, M.; Mayer, G. A New Saponin Lactone from Astragalus Corniculatus. Nat. Prod. Res. 2007, 21, 941–945. [Google Scholar] [CrossRef]
- Krasteva, I.N.; Toshkova, R.A.; Nikolov, S.D. Protective Effect of Astragalus Corniculatus Saponins against Myeloid Graffi Tumour in Hamsters. Phytother. Res. 2004, 18, 255–257. [Google Scholar] [CrossRef]
- Toshkova, R.A.; Krasteva, I.N.; Wesselinova, D.W.; Nikolov, S.D. Influence of Purified Saponin Mixture from Astragalus Corniculatus Bieb. on Phagocytic Cells in Graffi-Tumor Bearing Hamsters. J. Ethnopharmacol. 2007, 109, 394–399. [Google Scholar] [CrossRef]
- Dineva, I.; Krasteva, I.; Berger, M.; Konstantinov, S. In Vitro Antineoplastic Activity of Some Cytoreductive Drugs versus New Compounds of Plant Origin. Intern. J. Curr. Chem. 2010, 1, 281–290. [Google Scholar]
- Du, M.; Wu, X.J.; Ding, J.; Hu, Z.B.; White, K.N.; Branford-White, C.J. Astragaloside IV and Polysaccharide Production by Hairy Roots of Astragalus Membranaceus in Bioreactors. Biotechnol. Lett. 2003, 25, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.A.; Roberts, S.C. Recent Advances towards Development and Commercialization of Plant Cell Culture Processes for the Synthesis of Biomolecules: Development and Commercialization of Plant Cell Culture. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I. Genetic Transformation in Astragalus spp. In Transgenic Medicinal Plants; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999; pp. 55–72. [Google Scholar]
- Ionkova, I.; Kartnig, T.; Alfermann, W. Cycloartane Saponin Production in Hairy Root Cultures of Astragalus Mongholicus. Phytochemistry 1997, 45, 1597–1600. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic Hairy Roots. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Ionkova, I. Optimization of Flavonoid Production in Cell Cultures of Astragalus Missouriensis Nutt. (Fabaceae). Pharmacogn. Mag. 2009, 5, 92–97. [Google Scholar]
- Zdraveva, P.; Popova, P.; Shkondrov, A.; Krasteva, I.; Ionkova, I. Investigation of in vitro cultures of Astragalus monspessulanus L. Comptes Rendus De L’académie Bulg. Des Sci. 2017, 70, 1131–1137. [Google Scholar]
- Bratkov, V.; Kondeva-Burdina, M.; Simeonova, R.; Tzankova, V.; Krasteva, I.; others. Phytochemical Evaluation and Effect of Saponins’ Mixture Isolated from Astragalus Monspessulanus on HepG2 Cell Line. Eur. J. Med. Plants 2014, 4, 522–527. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Ma, W.; Yao, L.-P.; Feng, C.; Xia, X.-X. Optimization of Astragalus Membranaceus Hairy Roots Induction and Culture Conditions for Augmentation Production of Astragalosides. Plant Cell Tissue Organ Cult. 2015, 120, 1117–1130. [Google Scholar] [CrossRef]
- Groussin, A.-L.; Antoniotti, S. Valuable Chemicals by the Enzymatic Modification of Molecules of Natural Origin: Terpenoids, Steroids, Phenolics and Related Compounds. Bioresour. Technol. 2012, 115, 237–243. [Google Scholar] [CrossRef]
- Toshkova, R.A.; Krasteva, I.N.; Nikolov, S.D. Immunorestoration and Augmentation of Mitogen Lymphocyte Response in Graffi Tumor Bearing Hamsters by Purified Saponin Mixture from Astragalus Corniculatus. Phytomedicine 2008, 15, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Fu, Y.-J.; Zu, Y.-G.; Wang, W.; Mu, F.-S.; Luo, M.; Li, C.-Y.; Gu, C.-B.; Zhao, C.-J. Biotransformation of Saponins to Astragaloside IV from Radix Astragali by Immobilized Aspergillus Niger. Biocatal. Agric. Biotechnol. 2013, 2, 196–203. [Google Scholar] [CrossRef]
- Popova, P.; Zarev, Y.; Ionkova, I. Biotransformation of Quercetin, Kaempferol and Apigenin to Monoglycosylated Derivatives by in Vitro Suspension Cultures of Astragalus Vesicarius Ssp. Carniolicus. Pharmacia 2021, 68, 307–311. [Google Scholar] [CrossRef]
- Ionkova, I.; Antonova, I.; Momekov, G.; Fuss, E. Production of Podophyllotoxin in Linum Linearifolium in Vitro Cultures. Pharmacogn. Mag. 2010, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Popova, P.; Zarev, Y.; Shkondrov, A.; Krasteva, I.; Ionkova, I. Induction of Flavonoid Biosynthesis by in Vitro Cultivation of Astragalus Glycyphyllos L. Pharmacia 2020, 67, 95–99. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, W.; Luo, M.; Zu, Y.-G.; Fu, Y.-J.; Ma, W. Enhanced Astragaloside Production and Transcriptional Responses of Biosynthetic Genes in Astragalus Membranaceus Hairy Root Cultures by Elicitation with Methyl Jasmonate. Biochem. Eng. J. 2016, 105, 339–346. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Ma, W. UV Elicitation for Promoting Astragaloside Production in Astragalus Membranaceus Hairy Root Cultures with Transcriptional Expression of Biosynthetic Genes. Ind. Crops Prod. 2016, 84, 350–357. [Google Scholar] [CrossRef]
- Ionkova, I.; Momekov, G.; Proksch, P. Effects of Cycloartane Saponins from Hairy Roots of Astragalus Membranaceus Bge., on Human Tumor Cell Targets. Fitoterapia 2010, 81, 447–451. [Google Scholar] [CrossRef]
- Ren, J.; Wang, J.; Li, M.; Wang, L. Identifying Protein Complexes Based on Density and Modularity in Protein-Protein Interaction Network. BMC Syst. Biol. 2013, 7, S12. [Google Scholar] [CrossRef]
- Yao, M.; Liu, J.-Z.; Jin, S.; Jiao, J.; Gai, Q.; Wei, Z.; Fu, Y.; Zhao, J. A Novel Biotransformation of Astragalosides to Astragaloside IV with the Deacetylation of Fungal Endophyte Penicillium Canescens. Process Biochem. 2014, 49, 807–812. [Google Scholar] [CrossRef]
- Ye, L.; Liu, X.-H.; Zhou, W.; Feng, M.-Q.; Shi, X.-L.; Li, J.-Y.; Chen, D.-F.; Zhou, P. Microbial Transformation of Astragalosides to Astragaloside IV by Absidia Corymbifera AS2. Process Biochem. 2011, 46, 1724–1730. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Khakimov, B.; Kuzina, V.; Erthmann, P.Ø.; Fukushima, E.O.; Augustin, J.M.; Olsen, C.E.; Scholtalbers, J.; Volpin, H.; Andersen, S.B.; Hauser, T.P.; et al. Identification and Genome Organization of Saponin Pathway Genes from a Wild Crucifer, and Their Use for Transient Production of Saponins in Nicotiana Benthamiana. Plant J. 2015, 84, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Faizal, A.; Apers, S.; Pieters, L.; Thevelein, J.M.; Geelen, D.; Goossens, A. Unraveling the Triterpenoid Saponin Biosynthesis of the African Shrub Maesa Lanceolata. Mol. Plant 2015, 8, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.B.; de S. Borges, W.; Durán-Patrón, R.; Pupo, M.T.; Bonato, P.S.; Collado, I.G. Stereoselective Biotransformations Using Fungi as Biocatalysts. Tetrahedron Asymmetry 2009, 20, 385–397. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial Biotransformation of Bioactive Flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.-E.-H.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial Biotransformation as a Tool for Drug Development Based on Natural Products from Mevalonic Acid Pathway: A Review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef]
- Zeng, W.-L.; Li, W.-K.; Han, H.; Tao, Y.-Y.; Yang, L.; Wang, Z.-T.; Chen, K.-X. Microbial Biotransformation of Gentiopicroside by the Endophytic Fungus Penicillium Crustosum 2T01Y01. Appl. Env. Microbiol. 2014, 80, 184–192. [Google Scholar] [CrossRef]
- Feng, C.; Jin, S.; Xia, X.-X.; Guan, Y.; Luo, M.; Zu, Y.-G.; Fu, Y.-J. Effective Bioconversion of Sophoricoside to Genistein from Fructus Sophorae Using Immobilized Aspergillus Niger and Yeast. World J. Microbiol. Biotechnol. 2015, 31, 187–197. [Google Scholar] [CrossRef]
- Jin, S.; Luo, M.; Wang, W.; Zhao, C.; Gu, C.; Li, C.; Zu, Y.; Fu, Y.; Guan, Y. Biotransformation of Polydatin to Resveratrol in Polygonum Cuspidatum Roots by Highly Immobilized Edible Aspergillus Niger and Yeast. Bioresour. Technol. 2013, 136, 766–770. [Google Scholar] [CrossRef]
- Karami, O.; Esna-Ashari, M.; Kurdistani, G.; Aghavaisi, B. Agrobacterium-Mediated Genetic Transformation of Plants: The Role of Host. Biol. Plant 2009, 53, 201–212. [Google Scholar] [CrossRef]
- Kanazawa, K.; Hashimoto, T.; Yoshida, S.; Sungwon, P.; Fukuda, S. Short Photoirradiation Induces Flavonoid Synthesis and Increases Its Production in Postharvest Vegetables. J. Agric. Food Chem. 2012, 60, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, D.; Guillén, F.; Pérez-Aguilar, H.; Castillo, S.; Serrano, M.; Zapata, P.J.; Valero, D. Is It Possible To Increase the Aloin Content of Aloe Vera by the Use of Ultraviolet Light? J. Agric. Food Chem. 2013, 61, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Li, X.-X.; Chu, Y.-N.; Zhang, M.-X.; Wen, Y.-Q.; Duan, C.-Q.; Pan, Q.-H. Three Types of Ultraviolet Irradiation Differentially Promote Expression of Shikimate Pathway Genes and Production of Anthocyanins in Grape Berries. Plant Physiol. Biochem. 2012, 57, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Che, X.-N.; Pan, Q.-H.; Li, X.-X.; Duan, C.-Q. Transcriptional Activation of Flavan-3-Ols Biosynthesis in Grape Berries by UV Irradiation Depending on Developmental Stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. The Genus Gloriosa (Colchicaceae): Ethnobotany, Phylogeny and Taxonomy; Wageningen University: Wageningen, The Netherlands, 2012. [Google Scholar]
- Samy, R.P.; Thwin, M.M.; Gopalakrishnakone, P.; Ignacimuthu, S. Ethnobotanical Survey of Folk Plants for the Treatment of Snakebites in Southern Part of Tamilnadu, India. J. Ethnopharmacol. 2008, 115, 302–312. [Google Scholar] [CrossRef]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M.P. New Ethnomedicinal Claims from Gujjar and Bakerwals Tribes of Rajouri and Poonch Districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef]
- Maroyi, A. Gloriosa Superba L. (Family Colchicaceae): Remedy or Poison? J. Med. Plants Res. 2011, 5, 6112–6121. [Google Scholar] [CrossRef]
- Ade, R.; Rai, M.K. Review: Current Advances in Gloriosa Superba L. Biodiversitas 2009, 10, 210–214. [Google Scholar] [CrossRef]
- Murugesan, A.K.; Pannerselvam, B.; Javee, A.; Rajenderan, M.; Thiyagarajan, D. Facile Green Synthesis and Characterization of Gloriosa Superba L. Tuber Extract-Capped Silver Nanoparticles (GST-AgNPs) and Its Potential Antibacterial and Anticancer Activities against A549 Human Cancer Cells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100460. [Google Scholar] [CrossRef]
- Gelmi, M.L.; Mottadelli, S.; Pocar, D.; Riva, A.; Bombardelli, E.; De Vincenzo, R.; Scambia, G. N-Deacetyl-N-Aminoacylthiocolchicine Derivatives: Synthesis and Biological Evaluation on MDR-Positive and MDR-Negative Human Cancer Cell Lines. J. Med. Chem. 1999, 42, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Prasad, S.; Phromnoi, K.; Ravindran, J.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Thiocolchicoside Exhibits Anticancer Effects through Downregulation of NF-ΚB Pathway and Its Regulated Gene Products Linked to Inflammation and Cancer. Cancer Prev. Res. 2010, 3, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, K.; Chen, X.; Brossi, A.; Verdier-Pinard, P.; Hamel, E.; McPhail, A.T.; Tropsha, A.; Lee, K.-H. Antitumor Agents. 183. Syntheses, Conformational Analyses, and Antitubulin Activity of Allothiocolchicinoids. J. Org. Chem. 1998, 63, 4018–4025. [Google Scholar] [CrossRef]
- Balkrishna, A.; Das, S.K.; Pokhrel, S.; Joshi, A.; Verma, S.; Sharma, V.K.; Sharma, V.; Sharma, N.; Joshi, C.S. Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa Superba Seeds. Molecules 2019, 24, 2772. [Google Scholar] [CrossRef]
- Lin, Z.-Y.; Kuo, C.-H.; Wu, D.-C.; Chuang, W.-L. Anticancer Effects of Clinically Acceptable Colchicine Concentrations on Human Gastric Cancer Cell Lines. Kaohsiung J. Med. Sci. 2016, 32, 68–73. [Google Scholar] [CrossRef]
- Riva, S.; Sennino, B.; Zambianchi, F.; Danieli, B.; Panza, L. Effect of Organic Cosolvents on the Stability and Activity of the β-1,4-Galactosyltransferase from Bovine Colostrum. Carbohydr. Res. 1997, 305, 525–531. [Google Scholar] [CrossRef]
- Pišvejcová, A.; Rossi, C.; Hušáková, L.; Křen, V.; Riva, S.; Monti, D. β-1,4-Galactosyltransferase-Catalyzed Glycosylation of Sugar Derivatives: Modulation of the Enzyme Activity by α-Lactalbumin, Immobilization and Solvent Tolerance. J. Mol. Catal. B: Enzym. 2006, 39, 98–104. [Google Scholar] [CrossRef]
- Saradha Devi, M.; Ashokkumar, K.; Annapoorani, S. Phytofabrication and Encapsulated of Silver Nanoparticles from Gloriosa Superba. Mater. Lett. 2017, 188, 197–200. [Google Scholar] [CrossRef]
- Mahendran, D.; Kavi Kishor, P.B.; Geetha, N.; Manish, T.; Sahi, S.V.; Venkatachalam, P. Efficient Antibacterial/Biofilm, Anti-Cancer and Photocatalytic Potential of Titanium Dioxide Nanocatalysts Green Synthesised Using Gloriosa Superba Rhizome Extract. J. Exp. Nanosci. 2021, 16, 11–30. [Google Scholar] [CrossRef]
- Olowofolahan, A.O.; Olorunsogo, O.O. Effect of Gloriosa Superba Linn (EEGS) on MPT and Monosodium Glutamate-Induced Proliferative Disorder Using Rat Model. J. Ethnopharmacol. 2021, 267, 113498. [Google Scholar] [CrossRef]
- Mamatha, H.; Farooqi, A.A.; Joshi, S.S.; Prasad, T.G. Pollen studies in gloriosa superba linn. Acta Hortic. 1993, 331, 371–376. [Google Scholar] [CrossRef]
- Samarajeewa, P.K. Clonal Propagation of Gloriosa Superba L. Indian J. Exp. Biol. 1993, 31, 719–720. [Google Scholar]
- Sivakumar, G.; Krishnamurthy, K.; Rajendran, T.D. Embryoidogenesis and Plant Regeneration from Leaf Tissue of Gloriosa Superba. Planta Med 2003, 69, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Shekhawat, G.S. Critical Review on Medicinally Potent Plant Species: Gloriosa Superba. Fitoterapia 2011, 82, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Gopinath, K. In Vitro Micropropagation Using Corm Bud Explants: An Endangered Medicinal Plant of Gloriosa Superba L. Asian J. Biotechnol. 2012, 4, 120–128. [Google Scholar] [CrossRef]
- Sivakumar, S.; Siva, G.; Sathish, S.; Prem Kumar, G.; Vigneswaran, M.; Vinoth, S.; Kumar, T.S.; Sathishkumar, R.; Jayabalan, N. Influence of Exogenous Polyamines and Plant Growth Regulators on High Frequency in Vitro Mass Propagation of Gloriosa Superba L. and Its Colchicine Content. Biocatal. Agric. Biotechnol. 2019, 18, 101030. [Google Scholar] [CrossRef]
- Mahendran, D.; Kavi Kishor, P.B.; Sreeramanan, S.; Venkatachalam, P. Enhanced Biosynthesis of Colchicine and Thiocolchicoside Contents in Cell Suspension Cultures of Gloriosa Superba L. Exposed to Ethylene Inhibitor and Elicitors. Ind. Crops Prod. 2018, 120, 123–130. [Google Scholar] [CrossRef]
- Ponzone, C.; Berlanda, D.; Donzelli, F.; Acquati, V.; Ciulla, R.; Negrini, A.; Rovati, M.; Evangelista, D.; Fata, E.; Ciceri, D.; et al. Biotransformation of Colchicinoids into Their Corresponding 3-O-Glucosyl Derivatives by Selected Strains of Bacillus Megaterium. Mol. Biotechnol. 2014, 56, 653–659. [Google Scholar] [CrossRef]
- Alkaloids Corporation. Process for the Conversion of Colchicinoids to Their 3-Glycosylated Derivatives via Their Respective 3-Demethyl Analogues. European Patent EP3086794B1, 8 January 2010.
- Poulev, A.; Bombardelli, E.; Ponzone, C.; Zenk, M.H. Regioselective Bioconversion of Colchicine and Thiocolchicine into Their Corresponding 3-Demethyl Derivatives. J. Ferment. Bioeng. 1995, 79, 33–38. [Google Scholar] [CrossRef]
- Solet, J.-M.; Bister-Miel, F.; Galons, H.; Spagnoli, R.; Guignard, J.-L.; Cosson, L. Glucosylation of Thiocolchicine by a Cell Suspension Culture of Centella Asiatica. Phytochemistry 1993, 33, 817–820. [Google Scholar] [CrossRef]
- Zarev, Y.; Popova, P.; Foubert, K.; Apers, S.; Vlietinck, A.; Pieters, L.; Ionkova, I. Biotransformation to Produce the Anticancer Compound Colchicoside Using Cell Suspension Cultures of Astragalus Vesicarius Plant Species. Nat. Prod. Commun. 2019, 14, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Yadav, K.; Aggarwal, A.; Singh, N. Evaluation of Genetic Fidelity among Micropropagated Plants of Gloriosa Superba L. Using DNA-Based Markers—A Potential Medicinal Plant. Fitoterapia 2013, 89, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Aggarwal, A.; Singh, N. Arbuscular Mycorrhizal Fungi (AMF) Induced Acclimatization, Growth Enhancement and Colchicine Content of Micropropagated Gloriosa Superba L. Plantlets. Ind. Crops Prod. 2013, 45, 88–93. [Google Scholar] [CrossRef]


| Astragalus (Incl. Astracantha) Species | Location | Health Disorders | Reference |
|---|---|---|---|
| Astragalus sp. | Turkey | Roots cooked with milk for poultice applied to abdomen | [35] |
| A. amblolepis Fisch | Turkey | Unspecified cancer | [41] |
| A. abolinii Popov | Uzbekistan | Kidney disease, hypertension, burns, demulcent | [32] |
| A. americanus (Hook.) M.E.Jones | American countries | Stomach pain and flu | [32] |
| A. amherstianus Benth. | Pakistan | Galactagogue in animals | [32] |
| A. amphioxys A.Gray | America countries | Rattlesnake bite | [32] |
| A. angustifolius Lam | Lebanon | Astringent | [32] |
| A. armatus Willd. | Algeria | Leishmaniasis, helminthiasis | [32] |
| A. brachycalyx Fisch. ex Boiss. | Iran | Laxative, febrifuge, and digestive | [32] |
| A. brachycalyx Fisch. ex Boiss | Turkey | Unspecified cancer | [41] |
| A. caucasicus Pall. | Turkey | Diabetes | [40] |
| A. caucasicus Pall. | Caucasus, Georgia | Food (tea) | [43] |
| A. canadensis | America countries | Analgesic | [32] |
| A. camptoceras Bunge | Iran | Cold | [32] |
| A. cephalotes Banks. & Sol. var. brevicalyx Eig. | Turkey | Diabetes, wound healing | [37] |
| A. coluteoides Willd. | Lebanon | Diabetes and jaundice | [32] |
| A. chamaephaca Freyn | Turkey | Mouth wounds | [39] |
| A. crassicarpus Nutt. | American countries | tonic, anticonvulsive and anti-headache | [32] |
| A. creticus Lam. | Pakistan | Sedative and tonic | [32] |
| A. crenatus Schult. | Iran | Kidney stone, sedative, arthrodynia, carminative | [32] |
| A. cruentiflorus Boiss. | Lebanon | Diabetes and jaundice | [32] |
| A. dasyanthus Pall. | Ukraine | Cardiovascular insufficiency and chronic nephritis | [44] |
| A. effusus Bunge | Iran | Cough | [32] |
| A. fasciculifolius Boiss. | Iran | Cough, kidney, stomach ache, chest infection, toothache | [32] |
| A. fischeri Buhse ex Fisch. | Iran | Toothache, backache, bone ache, kidney ache, bone fracture, and diabetes, and to induce abortion | [32] |
| A. glaucacanthos Fisch. | Iran | Tonic, gastric pain, headache | [32] |
| A. globiflorus Boiss. | Iran | Healing deep infectious wounds | [32] |
| A. glycyphyllos L. | Bulgaria | Abdominal pain, colic, renal inflammation, menstrual disorders, and sciatica | [33] |
| A. glycyphyllos L. | Montenegro | Increasing men’s sexual potency | [32] |
| A. glycyphyllos L. | Italy | Diuretic, kidney ailments, gout, and rheumatism. | [32] |
| A. gossypinus Fisch. | Iran | Cough | [32] |
| A. grahamianus Benth. | Pakistan | Treatment of abscesses and as an analgesic | [32] |
| A. gummifer Lab. | Turkey | Throat diseases | [36] |
| A. gummifer Lab. | Turkey | Diabetes | [38] |
| A. hamosus L. | India | Nervous system disorders; liver, kidney, and spleen infection. | [32] |
| A. jolderensis B.Fedtsch. | Iran | Typhoid and dermal problems | [32] |
| A. lamarckii Boiss. | Turkey | Ulcer | [32] |
| A. longifolius Lam. | Turkey | Cardiac disorder, diabetes | [38] |
| A. microcephalus Willd. | Turkey | Unspecified cancer | [41] |
| A. microcephalus Willd. | Iran | Asthma, strengthen hair | [32] |
| A. mongholicus Bunge | China | Qi (Chi) tonic | [29,30] |
| A. mongholicus Bunge | China | Cancer | [45,46,47] |
| A. monspessulanus L. | Italy | Diuretic | [32] |
| A. mucronifolius Boiss. | Iran | Backache | [32] |
| A. noaeanus Boiss. | Turkey | Varicosis | [32] |
| A. ovinus Boiss. | Iran | Stomachache | [32] |
| A. tragalus podolobus Boiss. & Hohen. | Iran | Abdominal pain | [32] |
| A. psilocentros Fisch. | Pakistan | Cataract and stomach problems | [32] |
| A. rhizanthus Benth. | India | Digestive disorders, leucorrhea, and urinary troubles | [32] |
| A. rubrivenosus Gontsch. | Uzbekistan | Kidney disease, hypertonic disease, burns, demulcent | [32] |
| A. sarcocolla Dymock | Jordan | Incense, pains | [32] |
| A. sieversianus Pall. | Iran | Menstrual disorders | [32] |
| A. spinosus Muschl. | Pakistan | To treat wounds | [48] |
| A. thomsonianus Benth. ex Bunge | India | Gastric troubles, swelling, and joint pains | [32] |
| A. tmoleus Boiss. | Turkey | Toothache | [32] |
| A. tribulifolius Bunge | India | Diuretic agent and to lower kidney disorders. | [32] |
| A. tribuloides Delile | Iran | Urinary infection | [32] |
| A. verus Olivier | Iran | Antiparasitic, antimycotic, and immunomodulatory activities | [32] |
| A. zanskarensis Bunge | India | Against worms | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionkova, I.; Shkondrov, A.; Zarev, Y.; Kozuharova, E.; Krasteva, I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Curr. Issues Mol. Biol. 2022, 44, 3884-3904. https://doi.org/10.3390/cimb44090267
Ionkova I, Shkondrov A, Zarev Y, Kozuharova E, Krasteva I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Current Issues in Molecular Biology. 2022; 44(9):3884-3904. https://doi.org/10.3390/cimb44090267
Chicago/Turabian StyleIonkova, Iliana, Aleksandar Shkondrov, Yancho Zarev, Ekaterina Kozuharova, and Ilina Krasteva. 2022. "Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L." Current Issues in Molecular Biology 44, no. 9: 3884-3904. https://doi.org/10.3390/cimb44090267
APA StyleIonkova, I., Shkondrov, A., Zarev, Y., Kozuharova, E., & Krasteva, I. (2022). Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Current Issues in Molecular Biology, 44(9), 3884-3904. https://doi.org/10.3390/cimb44090267