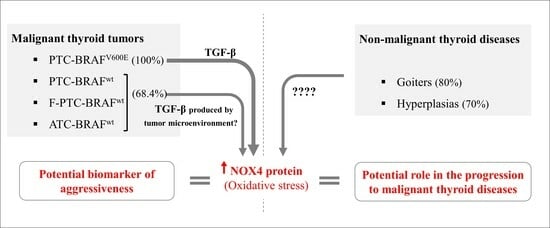
A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. NOX4 Immunohistochemistry Staining
2.3. Mutational Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of Patients
3.2. Clinicopathological Parameters of Each Histological Subtype of Thyroid Carcinomas
3.3. BRAFV600E Mutation in Thyroid Carcinomas
3.4. NOX4 Protein Expression in Human Malignant Thyroid Tissues
3.5. NOX4 Protein Expression and Subcellular Localization in Non-Malignant Human Thyroid Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular Genetics and Diagnosis of Thyroid Cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yuan, I.J.; Mirshahidi, S.; Simental, A.; Lee, S.C.; Yuan, X. Thyroid Carcinoma: Phenotypic Features, Underlying Biology and Potential Relevance for Targeting Therapy. Int. J. Mol. Sci. 2021, 22, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kaabouch, M.; Chahdi, H.; Azouzi, N.; Oukabli, M.; Rharrassi, I.; Boudhas, A.; Jaddi, H.; Ababou, M.; Dakka, N.; Boichard, A.; et al. BRAFV600E Hot Spot Mutation in Thyroid Carcinomas: First Moroccan Experience from a Single-Institution Retrospective Study. Afr. Health Sci. 2020, 20, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Bychkov, A.; Jung, C.-K. Emerging Biomarkers in Thyroid Practice and Research. Cancers 2021, 14, 204. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Schechter, R.B.; Shih, Y.-C.T.; Kaplan, E.L.; Chiu, B.C.H.; Angelos, P.; Grogan, R.H. The Clinical and Economic Burden of a Sustained Increase in Thyroid Cancer Incidence. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1252–1259. [Google Scholar] [CrossRef]
- Kilfoy, B.A.; Zheng, T.; Holford, T.R.; Han, X.; Ward, M.H.; Sjodin, A.; Zhang, Y.; Bai, Y.; Zhu, C.; Guo, G.L.; et al. International Patterns and Trends in Thyroid Cancer Incidence, 1973–2002. Cancer Causes Control 2009, 20, 525–531. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Giordano, T.J. Follicular Cell Thyroid Neoplasia: Insights from Genomics and The Cancer Genome Atlas Research Network. Curr. Opin. Oncol. 2016, 28, 1–4. [Google Scholar] [CrossRef]
- Jung, C.K.; Little, M.P.; Lubin, J.H.; Brenner, A.V.; Wells, S.A.; Sigurdson, A.J.; Nikiforov, Y.E. The Increase in Thyroid Cancer Incidence during the Last Four Decades Is Accompanied by a High Frequency of BRAF Mutations and a Sharp Increase in RAS Mutations. J. Clin. Endocrinol. Metab. 2014, 99, E276–E285. [Google Scholar] [CrossRef]
- Newfield, R.S.; Jiang, W.; Sugganth, D.X.; Hantash, F.M.; Lee, E.; Newbury, R.O. Mutational Analysis Using Next Generation Sequencing in Pediatric Thyroid Cancer Reveals BRAF and Fusion Oncogenes Are Common. Int. J. Pediatr. Otorhinolaryngol. 2022, 157, 111121. [Google Scholar] [CrossRef]
- Nikita, M.E.; Jiang, W.; Cheng, S.-M.; Hantash, F.M.; McPhaul, M.J.; Newbury, R.O.; Phillips, S.A.; Reitz, R.E.; Waldman, F.M.; Newfield, R.S. Mutational Analysis in Pediatric Thyroid Cancer and Correlations with Age, Ethnicity, and Clinical Presentation. Thyroid 2016, 26, 227–234. [Google Scholar] [CrossRef]
- Pérez-Fernández, L.; Sastre, J.; Zafón, C.; Oleaga, A.; Castelblanco, E.; Capel, I.; Galofré, J.C.; Guadalix-Iglesias, S.; De la Vieja, A.; Riesco-Eizaguirre, G. Validation of Dynamic Risk Stratification and Impact of BRAF in Risk Assessment of Thyroid Cancer, a Nation-Wide Multicenter Study. Front. Endocrinol. 2023, 13, 1071775. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.-Y.; Shibru, D.; Bastian, B.; Griffin, A. The Prevalence and Prognostic Value of BRAF Mutation in Thyroid Cancer. Ann. Surg. 2007, 246, 466–471. [Google Scholar] [CrossRef]
- Kimura, E.T.; Nikiforova, M.N.; Zhu, Z.; Knauf, J.A.; Nikiforov, Y.E.; Fagin, J.A. High Prevalence of BRAF Mutations in Thyroid Cancer: Genetic Evidence for Constitutive Activation of the RET/PTC-RAS-BRAF Signaling Pathway in Papillary Thyroid Carcinoma. Cancer Res. 2003, 63, 1454–1457. [Google Scholar]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients with Papillary Thyroid Cancer. JAMA 2013, 309, 1493. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef]
- Azouzi, N.; Cailloux, J.; Cazarin, J.M.; Knauf, J.A.; Cracchiolo, J.; Al Ghuzlan, A.; Hartl, D.; Polak, M.; Carré, A.; El Mzibri, M.; et al. NADPH Oxidase NOX4 Is a Critical Mediator of BRAF V600E—Induced Downregulation of the Sodium/Iodide Symporter in Papillary Thyroid Carcinomas. Antioxid. Redox Signal. 2017, 26, 864–877. [Google Scholar] [CrossRef]
- Ieni, A.; Vita, R.; Pizzimenti, C.; Benvenga, S.; Tuccari, G. Intratumoral Heterogeneity in Differentiated Thyroid Tumors: An Intriguing Reappraisal in the Era of Personalized Medicine. J. Pers. Med. 2021, 11, 333. [Google Scholar] [CrossRef]
- Bangaraiahgari, R.; Panchangam, R.B.; Puthenveetil, P.; Mayilvaganan, S.; Bangaraiahgari, R.; Reddy Banala, R.; Karunakaran, P.; Md, R. Is There Adenoma-Carcinoma Sequence between Benign Adenoma and Papillary Cancer of Thyroid: A Genomic Linkage Study. Ann. Med. Surg. 2020, 60, 695–700. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, W.Y.; Hwang, T.S.; Lee, S.S.; Kim, H.; Han, H.S.; Lim, S.D.; Kim, W.S.; Yoo, Y.B.; Park, K.S. BRAF and RAS Mutations in Follicular Variants of Papillary Thyroid Carcinoma. Endocr. Pathol. 2013, 24, 69–76. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yoshida, H.; Maruo, R.; Morita, S.; Takano, T.; Hirokawa, M.; Yabuta, T.; Fukushima, M.; Inoue, H.; Tomoda, C.; et al. BRAF Mutation in Papillary Thyroid Carcinoma in a Japanese Population: Its Lack of Correlation with High-Risk Clinicopathological Features and Disease-Free Survival of Patients. Endocr. J. 2009, 56, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Trovisco, V.; Soares, P.; Preto, A.; De Castro, I.V.; Lima, J.; Castro, P.; Máximo, V.; Botelho, T.; Moreira, S.; Meireles, A.M.; et al. Type and Prevalence of BRAF Mutations Are Closely Associated with Papillary Thyroid Carcinoma Histotype and Patients’ Age but Not with Tumour Aggressiveness. Virchows Arch. 2005, 446, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, D.; Incensati, R.M.; Materazzi, G.; Boni, G.; Grosso, M.; Panicucci, E.; Lapi, P.; Pasquini, C.; Miccoli, P. The BRAF V600E Mutation in Papillary Thyroid Cancer with Positive or Suspected Pre-Surgical Cytological Finding Is Not Associated with Advanced Stages or Worse Prognosis. Endocrine 2014, 45, 462–468. [Google Scholar] [CrossRef]
- Gandolfi, G.; Sancisi, V.; Torricelli, F.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A. Allele Percentage of the BRAF V600E Mutation in Papillary Thyroid Carcinomas and Corresponding Lymph Node Metastases: No Evidence for a Role in Tumor Progression. J. Clin. Endocrinol. Metab. 2013, 98, E934–E942. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Weyemi, U.; Caillou, B.; Talbot, M.; Ameziane-El-Hassani, R.; Lacroix, L.; Lagent-Chevallier, O.; Al Ghuzlan, A.; Roos, D.; Bidart, J.-M.; Virion, A.; et al. Intracellular Expression of Reactive Oxygen Species-Generating NADPH Oxidase NOX4 in Normal and Cancer Thyroid Tissues. Endocr. Relat. Cancer 2010, 17, 27–37. [Google Scholar] [CrossRef]
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. [Google Scholar] [CrossRef]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta Histochem. Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Ofner, R.; Ritter, C.; Ugurel, S.; Cerroni, L.; Stiller, M.; Bogenrieder, T.; Solca, F.; Schrama, D.; Becker, J.C. Non-Reproducible Sequence Artifacts in FFPE Tissue: An Experience Report. J. Cancer Res. Clin. Oncol. 2017, 143, 1199–1207. [Google Scholar] [CrossRef]
- Wong, S.Q.; Li, J.; Tan, A.Y.C.; Vedururu, R.; Pang, J.M.B.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; Macarthur, G.A.; et al. Sequence Artefacts in a Prospective Series of Formalin-Fixed Tumours Tested for Mutations in Hotspot Regions by Massively Parallel Sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef]
- Cazzato, G.; Caporusso, C.; Arezzo, F.; Cimmino, A.; Colagrande, A.; Loizzi, V.; Cormio, G.; Lettini, T.; Maiorano, E.; Scarcella, V.; et al. Formalin-Fixed and Paraffin-Embedded Samples for Next Generation Sequencing: Problems and Solutions. Genes 2021, 12, 1472. [Google Scholar] [CrossRef]
- Ameziane-El-Hassani, R.; Schlumberger, M.; Dupuy, C. NADPH Oxidases: New Actors in Thyroid Cancer? Nat. Rev. Endocrinol. 2016, 12, 485–494. [Google Scholar] [CrossRef]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative Stress in Thyroid Carcinomas: Biological and Clinical Significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Ameziane-El-Hassani, R.; Talbot, M.; de Souza Dos Santos, M.C.; Al Ghuzlan, A.; Hartl, D.; Bidart, J.-M.; De Deken, X.; Miot, F.; Diallo, I.; de Vathaire, F.; et al. NADPH Oxidase DUOX1 Promotes Long-Term Persistence of Oxidative Stress after an Exposure to Irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, 5051–5056. [Google Scholar] [CrossRef]
- Weyemi, U.; Lagente-Chevallier, O.; Boufraqech, M.; Prenois, F.; Courtin, F.; Caillou, B.; Talbot, M.; Dardalhon, M.; Al Ghuzlan, A.; Bidart, J.-M.; et al. ROS-Generating NADPH Oxidase NOX4 Is a Critical Mediator in Oncogenic H-Ras-Induced DNA Damage and Subsequent Senescence. Oncogene 2012, 31, 1117–1129. [Google Scholar] [CrossRef]
- Riesco-Eizaguirre, G.; Rodríguez, I.; De la Vieja, A.; Costamagna, E.; Carrasco, N.; Nistal, M.; Santisteban, P. The BRAFV600E Oncogene Induces Transforming Growth Factor β Secretion Leading to Sodium Iodide Symporter Repression and Increased Malignancy in Thyroid Cancer. Cancer Res. 2009, 69, 8317–8325. [Google Scholar] [CrossRef]
- Rabold, K.; Zoodsma, M.; Grondman, I.; Kuijpers, Y.; Bremmers, M.; Jaeger, M.; Zhang, B.; Hobo, W.; Bonenkamp, H.J.; de Wilt, J.H.W.; et al. Reprogramming of Myeloid Cells and Their Progenitors in Patients with Non-Medullary Thyroid Carcinoma. Nat. Commun. 2022, 13, 6149. [Google Scholar] [CrossRef]
- Smith, J.J.; Chen, X.; Schneider, D.F.; Broome, J.T.; Sippel, R.S.; Chen, H.; Solórzano, C.C. Cancer after Thyroidectomy: A Multi-Institutional Experience with 1523 Patients. J. Am. Coll. Surg. 2013, 216, 571–577. [Google Scholar] [CrossRef]
- de Carlos, J.; Ernaga, A.; Irigaray, A.; Pineda, J.J.; Echegoyen, A.; Salvador, P.; Anda, E. Incidentally Discovered Papillary Thyroid Microcarcinoma in Patients Undergoing Thyroid Surgery for Benign Disease. Endocrine 2022, 77, 325–332. [Google Scholar] [CrossRef]
- Führer, D. Constitutive TSH Receptor Activation as a Hallmark of Thyroid Autonomy. Endocrine 2020, 68, 274–278. [Google Scholar] [CrossRef] [PubMed]




| Clinicopathological Parameters | n (%) |
|---|---|
| Gender | |
| Female | 39 (81%) |
| Male | 9 (19%) |
| Age | |
| Median ± standard error | 42.5 ± 2.4 |
| Histological variant | |
| C-PTC | 28/48 (58.33%) |
| F-PTC | 17/48 (35.41%) |
| ATC | 3/48 (6.26%) |
| Tumor size | |
| ≤1 cm | 7/48 (15%) |
| >1 cm | 30/48 (63%) |
| unknown | 11/48 (23%) |
| vascular emboli | |
| Presence | 7/48 (15%) |
| Absence | 41/48 (85%) |
| Capsular breach | |
| Presence | 11/48 (23%) |
| Absence | 37/48 (77%) |
| lymph node metastasis | |
| Presence | 3/48 (6.3%) |
| Absence | 34/48 (70.8%) |
| Unknown | 11/48 (22.9%) |
| Clinicopathological Parameters | PTC (n = 45) | C-PTC (n = 28) | F-PTC (n = 17) | ATC (n = 3) | C-PTC-F-PTC Chi-Square/Fisher’s Exact Test (p-Value < 0.05) |
|---|---|---|---|---|---|
| Age | 0.1516 | ||||
| <45 | 19/45 (42.2%) | 10/28 (35.7%) | 9/17 (52.9%) | 1/3 (33.3%) | |
| ≥45 | 16/45 (35.6%) | 13/28 (46.4%) | 3/17 (17.7%) | 2/3 (66.7%) | |
| unknown | 10/45 (22.2%) | 5/28 (17.9%) | 5/17 (29.4%) | - | |
| Tumor size | >0.9999 | ||||
| ≤1 cm | 7/45 (15.6%) | 4/28 (14.3%) | 3/17 (17.6%) | - | |
| >1 cm | 30/45 (66.7%) | 18/28 (64.3%) | 12/17 (70.6%) | - | |
| Unknown | 8/45 (17.7%) | 6/28 (21.4%) | 2/17 (11.8%) | 3 (100%) | |
| vascular emboli | 0.3846 | ||||
| Presence | 6/45 (13.3%) | 5/28 (17.9%) | 1/17 (5.9%) | 1/3 (33.3%) | |
| Absence | 39/45 (86.7%) | 23/28 (82.1%) | 16/17 (94.1%) | 2/3 (66.7%) | |
| Capsular breach | 0.1647 | ||||
| Presence | 11/45 (24.4%) | 9/28 (32.1%) | 2/17 (11.8%) | 0 (0%) | |
| Absence | 34/45 (75.6%) | 19/28 (67.9%) | 15/17 (88.2%) | 3/3 (100%) | |
| lymph node metastasis | 0.2432 | ||||
| Presence | 3/45 (6.6%) | 3/28 (10.7%) | 0 (0%) | 0 (0%) | |
| Absence | 34/45 (75.6%) | 18/28 (64.2%) | 16/17 (94.1%) | 0 (0%) | |
| Unknown | 8/45 (17.8%) | 7/28 (25%) | 1/17 (5.9%) | 3/3 (100%) |
| Clinicopathological Parameters | C-PTC BRAFV600E (n = 10) | C-PTC BRAFwt (n = 18) | Total | Chi-Square/Fisher’s Exact Test (p-Value < 0.05) |
|---|---|---|---|---|
| Tumor size | >0.9999 | |||
| ≤1 cm | 1/10 (10%) | 2/18 (11.1%) | 3 | |
| >1 cm | 7/10 (70%) | 12/18 (66.7%) | 19 | |
| unknown | 2/10 (20%) | 4/18 (22.2%) | 6 | |
| lymph node metastasis | >0.9999 | |||
| Presence | 1/10 (10%) | 2/18 (11.1%) | 3 | |
| Absence | 8/10 (80%) | 10/18 (55.6%) | 18 | |
| unknown | 1/10 (10%) | 6/18 (33.3%) | 7 | |
| vascular emboli | 0.8253 | |||
| Presence | 2/10 (20%) | 3/18 (16.7%) | 5 | |
| Absence | 8/10 (80%) | 15/18 (83.3%) | 23 | |
| Capsular breach | 0.5070 | |||
| Presence | 4/10 (40%) | 5/18 (27.8%) | 9 | |
| Absence | 6/10 (60%) | 13/18 (72.2%) | 19 |
| Clinicopathological Parameters | n (%) | NOX4 Overexpression (Score: ≥2) | NOX4 Low Expression (Score: <2) | Fisher’s Exact Test (p-Value < 0.05) |
|---|---|---|---|---|
| Gender | >0.9999 | |||
| Female | 39/48 (81%) | 30/39 (76.9%) | 9/39 (23.1%) | |
| Male | 9/48 (19%) | 7/9 (77.8%) | 2/9 (22.2%) | |
| Histological variant | <0.0001 | |||
| C-PTC | 28/48 (58.33%) | 26/28 (92.9%) | 2/28 (7.1%) | |
| F-PTC | 17/48 (35.41%) | 9/17 (52.9%) | 8/17 (47.1%) | |
| ATC | 3/48 (6.26%) | 1/3 (33.3%) | 2/3 (66.7%) | |
| Tumor size | 0.3079 | |||
| ≤1 cm | 7/48 (15%) | 7/7 (100%) | 0 (0%) | |
| >1 cm | 30/48 (63%) | 22/30 (73.3%) | 8/30 (26.7%) | |
| unknown | 11/48 (23%) | 7/11 (63.4%) | 4/11 (36.6%) | |
| vascular emboli | ||||
| Presence | 7/48 (15%) | 6/7 (85.7%) | 1/7 (14.3%) | |
| Absence | 41/48 (85%) | 30/41 (73.2%) | 11/41 (26.8%) | 0.6621 |
| Capsular breach | ||||
| Presence | 11/48 (23%) | 11/11 (100%) | 0/11 (0%) | |
| Absence | 37/48 (77%) | 25/37 (67.6%) | 12/37 (32.4%) | 0.0442 |
| lymph node metastasis | 0.5483 | |||
| Presence | 3/48 (6.3%) | 3/3 (100%) | 0/3 (0%) | |
| Absence | 34/48 (70.8%) | 24/34 (70.6%) | 10/34 (29.4%) | |
| unknown | 11/48 (22.9%) | 9/11 (81.8%) | 2/11 (18.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenniche, S.; Oukabli, M.; Oubaddou, Y.; Chahdi, H.; Damiri, A.; Alghuzlan, A.; Laraqui, A.; Dakka, N.; Bakri, Y.; Dupuy, C.; et al. A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors. Curr. Issues Mol. Biol. 2023, 45, 5811-5823. https://doi.org/10.3390/cimb45070367
Fenniche S, Oukabli M, Oubaddou Y, Chahdi H, Damiri A, Alghuzlan A, Laraqui A, Dakka N, Bakri Y, Dupuy C, et al. A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors. Current Issues in Molecular Biology. 2023; 45(7):5811-5823. https://doi.org/10.3390/cimb45070367
Chicago/Turabian StyleFenniche, Salma, Mohamed Oukabli, Yassire Oubaddou, Hafsa Chahdi, Amal Damiri, Abir Alghuzlan, Abdelilah Laraqui, Nadia Dakka, Youssef Bakri, Corinne Dupuy, and et al. 2023. "A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors" Current Issues in Molecular Biology 45, no. 7: 5811-5823. https://doi.org/10.3390/cimb45070367
APA StyleFenniche, S., Oukabli, M., Oubaddou, Y., Chahdi, H., Damiri, A., Alghuzlan, A., Laraqui, A., Dakka, N., Bakri, Y., Dupuy, C., & Ameziane El Hassani, R. (2023). A Comparative Analysis of NOX4 Protein Expression in Malignant and Non-Malignant Thyroid Tumors. Current Issues in Molecular Biology, 45(7), 5811-5823. https://doi.org/10.3390/cimb45070367