The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Surgical Procedure
2.3. Electrophysiological Recordings
2.4. Assessment of Motor Function via Inclined Plane Test
2.5. Histology and Quantitative Histochemistry
2.6. Nerve Biochemical Analysis
2.7. Statistical Analysis
3. Results
- Electrophysiological Assessments:
- Histological and Immunohistochemical Analysis:
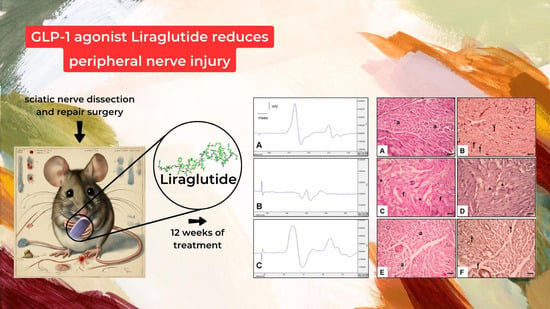
- Histological Assessment (Figure 3):
- Figure 3A,B: Control Group:
- Figure 3C,D: Surgery and Saline Group:
- Figure 3E,F: Surgery and Liraglutide Group:
- Nerve Biochemical Analysis:
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R. Peripheral nerve injury: A review and approach to tissue engineered constructs. Anat. Rec. 2001, 263, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Braza, D.; Rice, J.B.; Dillingham, T. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil. 2008, 87, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Kuyucu, E.; Gümüs, B.; Erbas, O.; Oltulu, F.; Bora, A. Exenatide promotes regeneration of injured rat sciatic nerve. Neural Regen. Res. 2017, 12, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, M.B.; Bora, E.S.; Erdoğan, M.A.; Çakır, A.; Erbaş, O. The Effect of Adipose-Derived Mesenchymal Stem Cells on Peripheral Nerve Damage in a Rodent Model. J. Clin. Med. 2023, 12, 6411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pradhan, S.; Liu, C.; Le, L. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells 2012, 30, 2261–2270. [Google Scholar] [CrossRef]
- Li, X.; Guan, Y.; Li, C.; Zhang, T.; Meng, F.; Zhang, J.; Li, J.; Chen, S.; Wang, Q.; Wang, Y.; et al. Immunomodulatory effects of mesenchymal stem cells in peripheral nerve injury. Stem Cell Res. Ther. 2022, 13, 18. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Zdravkovic, M.; Le-Thi, T.; Krarup, T.; Schmitz, O.; Courrèges, J.P.; Verhoeven, R.; Bugánová, I.; Madsbad, S. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007, 30, 1608–1610. [Google Scholar] [CrossRef]
- Sango, K.; Takaku, S.; Tsukamoto, M.; Niimi, N.; Yako, H. Glucagon- Like Peptide-1 Receptor Agonists as Potential Myelination-Inducible and Anti-Demyelinating Remedies. Front. Cell Dev. Biol. 2022, 10, 950623. [Google Scholar] [CrossRef]
- Harkavyi, A.; Whitton, P.S. Glucagon-like Peptide 1 Receptor Stimulation as a Means of Neuroprotection. Br. J. Pharmacol. 2010, 159, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jin, H.Y.; Lee, K.A.; Xie, S.H.; Baek, H.S.; Park, T.S. Neuroprotective Effect of the Glucagon-like Peptide-1 Receptor Agonist, Synthetic Exendin-4, in Streptozotocin-Induced Diabetic Rats. Br. J. Pharmacol. 2011, 164, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Bassil, F.; Canron, M.H.; Vital, A.; Bezard, E.; Li, Y.; Greig, N.H.; Gulyani, S.; Kapogiannis, D.; Fernagut, P.O.; Meissner, W.G. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain 2017, 140, 1420–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Tian, X. The pleiotropic of GLP-1/GLP-1R axis in central nervous system diseases. Int. J. Neurosci. 2023, 133, 473–491. [Google Scholar] [CrossRef]
- Hölscher, C. Protective Properties of GLP-1 and Associated Peptide Hormones in Neurodegenerative Disorders. Br. J. Pharmacol. 2022, 179, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C. Evaluating the Effects of the Novel GLP-1 Analogue Liraglutide in Alzheimer’s Disease: Study Protocol for a Randomised Controlled Trial (ELAD Study). Trials 2019, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Vijiaratnam, N.; Girges, C.; Auld, G.; Chau, M.; Maclagan, K.; King, A. Exenatide once Weekly over 2 Years as a Potential Disease-Modifying Treatment for Parkinson’s Disease: Protocol for a Multicentre, Randomised, Double Blind, Parallel Group, Placebo Controlled, Phase 3 Trial: The ‘Exenatide-PD3’ Study. BMJ Open 2019, 11, e047993. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Machelak, W.; Szczepaniak, A.; Jacenik, D.; Zielińska, M. The role of GDF11 during inflammation—An overview. Life Sci. 2023, 322, 121650. [Google Scholar] [CrossRef]
- Smith, A.W.; Ray, S.K.; Das, A.; Nozaki, K.; Rohrer, B.; Banik, N.L. Calpain inhibition as a possible new therapeutic target in multiple sclerosis. AIMS Mol. Sci. 2017, 4, 446–462. [Google Scholar] [CrossRef]
- Shizuka, T.; Naoko, N.; Toshihiko, K.; Hideji, Y.; Masami, T.; Kunihiko, S.; Yusaku, N.; Hidenori, H.; Kazunori, S. Galectin-1 and galectin-3 as key molecules for peripheral nerve degeneration and regeneration. AIMS Mol. Sci. 2016, 3, 325–337. [Google Scholar]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Saada, A.; Reichert, F.; Rotshenker, S. Granulocyte macrophage colony stimulating factor produced in lesioned peripheral nerves induces the up-regulation of cell surface expression of MAC-2 by macrophages and Schwann cells. J. Cell Biol. 1996, 133, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Taşkıran, E.; Erdoğan, M.A.; Yiğittürk, G.; Erbaş, O. Therapeutic Effects of Liraglutide, Oxytocin and Granulocyte Colony-Stimulating Factor in Doxorubicin-Induced Cardiomyopathy Model: An Experimental Animal Study. Cardiovasc. Toxicol. 2019, 19, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jones, S.; Jia, X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kameda, M.; Yasuhara, T.; Agari, T.; Baba, T.; Wang, F.; Shinko, A.; Wakamori, T.; Toyoshima, A.; Takeuchi, H.; et al. Neuroprotective Effects of Liraglutide for Stroke Model of Rats. Int. J. Mol. Sci. 2013, 14, 21513–21524. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Mao, Y.; Zheng, Z.; Chen, Y.; Khor, S.; Shi, K.; He, Z.; Li, J.; Gong, F.; et al. Liraglutide activates autophagy via GLP-1R to improve functional recovery after spinal cord injury. Oncotarget 2017, 8, 85949–85968. [Google Scholar] [CrossRef]
- Steven, S.; Jurk, K.; Kopp, M.; Kroller-Schon, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br. J. Pharmacol. 2017, 174, 1620–1632. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Baek, G.H.; Oh, J.H.; Lee, Y.H.; Bin, S.W.; Gong, H.S. The effect of muscle length and excursion on muscle contracture after tendon injury: A study in rabbit soleus muscles. Injury 2007, 38, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Amako, M.; Yamamoto, Y.; Tsuchihara, T.; Nukada, H.; Yoshihara, Y.; Arino, H.; Fujita, M.; Uenoyama, M.; Tachibana, S.; et al. Therapeutic effect of exendin-4, a long-acting analogue of glucagon-like peptide-1 receptor agonist, on nerve regeneration after the crush nerve injury. BioMed Res. Int. 2013, 2013, 315848. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Siegmund, A.; Eigenberger, A.; Hartmann, V.; Langewost, F.; Hammer, N.; Anker, A.; Klein, K.; Morsczeck, C.; Prantl, L.; et al. Peripheral Nerve Regeneration-Adipose-Tissue-Derived Stem Cells Differentiated by a Three-Step Protocol Promote Neurite Elongation via NGF Secretion. Cells 2022, 11, 2887. [Google Scholar] [CrossRef]
- Xavier, A.M.; Serafim, K.G.; Higashi, D.T.; Vanat, N.; Flaiban, K.K.; Siqueira, C.P.; Venâncio, E.J.; Ramos Sde, P. Simvastatin improves morphological and functional recovery of sciatic nerve injury in Wistar rats. Injury 2012, 43, 284–289. [Google Scholar] [CrossRef]
- Hobbenaghi, R.; Javanbakht, J.; Sadeghzadeh, S.; Kheradmand, D.; Abdi, F.S.; Jaberi, M.H.; Mohammadiyan, M.R.; Khadivar, F.; Mollaei, Y. Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia-reperfusion injury in rats. J. Neurol. Sci. 2014, 337, 74–79. [Google Scholar] [CrossRef]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Gustavsson, P.; Linsmeier, C.E.; Leffler, H.; Kanje, M. Galectin-3 inhibits Schwann cell proliferation in cultured sciatic nerve. NeuroReport 2007, 18, 669–673. [Google Scholar] [CrossRef]
- Narciso, M.; Mietto, B.; Marques, S.; Soares, C.; Mermelstein, C.; El-Cheikh, M.; Martinez, A. Sciatic nerve regeneration is accelerated in galectin-3 knockout mice. Exp. Neurol. 2009, 217, 7–15. [Google Scholar] [CrossRef]
- Lin, J.; Shi, J.; Min, X.; Chen, S.; Zhao, Y.; Zhang, Y.; Cheng, L. The GDF11 Promotes Nerve Regeneration After Sciatic Nerve Injury in Adult Rats by Promoting Axon Growth and Inhibiting Neuronal Apoptosis. Front. Bioeng. Biotechnol. 2022, 9, 803052. [Google Scholar] [CrossRef]
- Tsai, M.J.; Fay, L.Y.; Liou, D.Y.; Chen, Y.; Chen, Y.T.; Lee, M.J.; Tu, T.H.; Huang, W.C.; Cheng, H. Multifaceted Benefits of GDF11 Treatment in Spinal Cord Injury: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2022, 24, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.H.; Peng, A.; Liu, X.Y.; Wang, Y.; Huang, S.H.; Liu, T.; Wang, X.J.; Chen, Z.Y. The neuroprotective and neurorestorative effects of growth differentiation factor 11 in cerebral ischemic injury. Brain Res. 2020, 15, 1737. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Xiang, G.; Li, Y.; Li, H.; Xiang, L.; Lu, J.; Xiang, L.; Dong, J.; Liu, M. GDF11 Protects against Endothelial Injury and Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Null Mice. Mol. Ther. 2016, 24, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.C.; Hehr, C.L.; Chang, R.Y.; Johnston, J.; McFarlane, S. TGFβ Ligands Promote the Initiation of Retinal Ganglion Cell Dendrites In Vitro and In Vivo. Mol. Cell Neurosci. 2008, 37, 247–260. [Google Scholar] [CrossRef]
- Augustin, H.; McGourty, K.; Steinert, J.R.; Cochemé, H.M.; Adcott, J.; Cabecinha, M.; Vincent, A.; Halff, E.F.; Kittler, J.T.; Boucrot, E.; et al. Myostatin-like proteins regulate synaptic function and neuronal morphology. Development 2017, 144, 2445–2455. [Google Scholar] [CrossRef]
- Moustafa, P.E.; Abdelkader, N.F.; El Awdan, S.A.; El-Shabrawy, O.A.; Zaki, H.F. Liraglutide ameliorated peripheral neuropathy in diabetic rats: Involvement of oxidative stress, inflammation and extracellular matrix remodeling. J. Neurochem. 2018, 146, 173–185. [Google Scholar] [CrossRef]



| Control | Surgery and Saline (Placebo) | Surgery and Liraglutide Group (Experimental Group) | |
|---|---|---|---|
| EMG CMAP latency (ms) | 2.25 ± 0.14 | 3.63 ± 0.11 * | 3.42 ± 0.15 |
| EMG CMAP amplitude (mV) | 13.3 ± 1.5 | 2.03 ± 0.2 ** | 8.3 ± 0.4 # |
| Inclaned plane score (°) | 82.6 ± 2.9 | 28.7 ± 6.4 ** | 77.1 ± 5.5 ## |
| Control | Surgery and Saline (Placebo) | Surgery and Liraglutide (Experimental) | |
|---|---|---|---|
| NGF immunoexpression on Schwann cell (%) | 91.4 ± 8.3 | 5.2 ± 0.6 ** | 73.6 ± 4.05 ## |
| Total axon number | 289.1 ± 16.3 | 17.6 ± 2.5 ** | 103.2 ± 9.2 ## |
| Axon diameter, µm | 3.46 ± 0.15 | 1.83 ± 0.24 * | 3.12 ± 0.17 # |
| Fibrosis score (%) | 0.8 ± 0.1 | 84.6 ± 7.5 ** | 7.5 ± 2.6 ## |
| Control | Surgery and Saline (Placebo) | Surgery and Liraglutide Group (Experimental Group) | |
|---|---|---|---|
| MDA (nmol/μg) | 103.1 ± 9.5 | 198.2 ± 10.6 * | 121.5 ± 8.1 # |
| Nerve Galectin-3 Level (pg/mg) | 6.3 ± 0.1 | 22.6 ± 0.9 ** | 10.4 ± 0.6 # |
| Nerve NGF Level (pg/mg) | 26.7 ± 0.5 | 10.1 ± 2.3 * | 21.2 ± 1.1 # |
| Nerve GDF-11 Level (pg/mg) | 17.8 ± 1.09 | 9.9 ± 0.5 * | 13.1 ± 1.6 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçın, M.B.; Bora, E.S.; Erbaş, O. The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion. Curr. Issues Mol. Biol. 2024, 46, 327-339. https://doi.org/10.3390/cimb46010021
Yalçın MB, Bora ES, Erbaş O. The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion. Current Issues in Molecular Biology. 2024; 46(1):327-339. https://doi.org/10.3390/cimb46010021
Chicago/Turabian StyleYalçın, Mehmet Burak, Ejder Saylav Bora, and Oytun Erbaş. 2024. "The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion" Current Issues in Molecular Biology 46, no. 1: 327-339. https://doi.org/10.3390/cimb46010021
APA StyleYalçın, M. B., Bora, E. S., & Erbaş, O. (2024). The Effect of Liraglutide on Axon Regeneration and Functional Recovery after Peripheral Nerve Lesion. Current Issues in Molecular Biology, 46(1), 327-339. https://doi.org/10.3390/cimb46010021