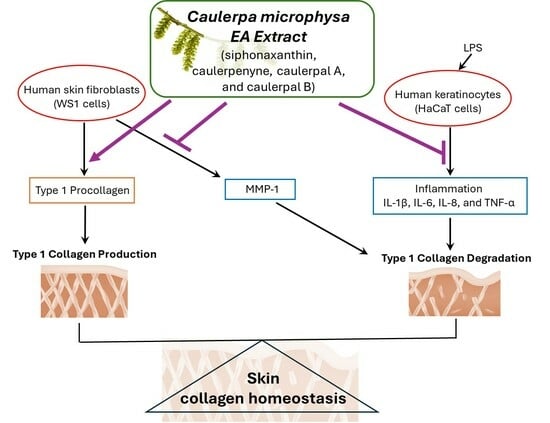
The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Preparation of Caulerpa microphysa Extracts
2.3. Ultra-Performance Liquid Chromatography (UPLC) and Quadrupole Time-of-Flight Mass Spectrometry (QTOF)
2.4. WST-1
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blot
2.7. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domozych, D.S.; LoRicco, J.G. The extracellular matrix of green algae. Plant Physiol. 2023, 194, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: From algae to flowering plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Stanton, A.; Archibald, J.M.; Gould, S.B. Streptophyte Terrestrialization in Light of Plastid Evolution. Trends Plant Sci. 2016, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–533. [Google Scholar]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 287–306. [Google Scholar]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–2667. [Google Scholar] [CrossRef]
- Hegazi, M.M.; Perez-Ruzafa, A.; Almela, L.; María-Emilia, C. Separation and identification of chlorophylls and carotenoids from Caulerpa prolifera, Jania rubens and Padina pavonica by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1998, 829, 153–159. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Domozych, D.S.; Bagdan, K. The cell biology of charophytes: Exploring the past and models for the future. Plant Physiol. 2022, 190, 1588–1608. [Google Scholar] [CrossRef]
- Kloareg, B.; Badis, Y.; Cock, J.M.; Michel, G. Role and Evolution of the Extracellular Matrix in the Acquisition of Complex Multicellularity in Eukaryotes: A Macroalgal Perspective. Genes 2021, 12, 1059. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Deudero, S.; Pons, A. Reciprocal effects of caulerpenyne and intense herbivorism on the antioxidant response of Bittium reticulatum and Caulerpa taxifolia. Ecotoxicol. Environ. Saf. 2009, 72, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Pei, Y.; Fang, Z.; Sun, P. Effects of partial desulfation on antioxidant and inhibition of DLD cancer cell of Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 2014, 65, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Chang, Y.C.; Denny, R.W. Chemistry of singlet oxygen. XI. Cis-trans isomerization of carotenoids by singlet oxygen and a probable quenching mechanism. J. Am. Chem. Soc. 1970, 92, 5218–5219. [Google Scholar] [CrossRef]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Manabe, Y.; Takii, Y.; Sugawara, T. Siphonaxanthin, a carotenoid from green algae, suppresses advanced glycation end product-induced inflammatory responses. J. Nat. Med. 2020, 74, 127–134. [Google Scholar] [CrossRef]
- Li, Z.S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef]
- Li, Z.S.; Zheng, J.W.; Manabe, Y.; Hirata, T.; Sugawara, T. Anti-Obesity Properties of the Dietary Green Alga, Codium cylindricum, in High-Fat Diet-Induced Obese Mice. J. Nutr. Sci. Vitaminol. 2018, 64, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Fischel, J.L.; Lemee, R.; Formento, P.; Caldani, C.; Moll, J.L.; Pesando, D.; Meinesz, A.; Grelier, P.; Pietra, P.; Guerriero, A.; et al. Cell growth inhibitory effects of caulerpenyne, a sesquiterpenoid from the marine algae Caulerpa taxifolia. Anticancer Res. 1995, 15, 2155–2160. [Google Scholar] [PubMed]
- Cavas, L.; Baskin, Y.; Yurdakoc, K.; Olgun, N. Antiproliferative and newly attributed apoptotic activities from an invasive marine alga: Caulerpa racemosa var. cylindracea. J. Exp. Mar. Biol. Ecol. 2006, 339, 111–119. [Google Scholar] [CrossRef]
- Nicoletti, E.; Della Pietà, F.; Calderone, V.; Bandecchi, P.; Pistello, M.; Morelli, I.; Cinelli, F. Antiviral properties of a crude extract from a green alga Caulerpa taxifolia (Vahl) C. Agardh. Phytother. Res. 1999, 13, 245–247. [Google Scholar] [CrossRef]
- Liu, A.H.; Liu, D.Q.; Liang, T.J.; Yu, X.Q.; Feng, M.T.; Yao, L.G.; Fang, Y.; Wang, B.; Feng, L.H.; Zhang, M.X.; et al. Caulerprenylols A and B, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorg. Med. Chem. Lett. 2013, 23, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yeh, H.Y.; Shih, W.L. Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Mar. Drugs 2021, 19, 524. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.S.P.; Costa, L.S.; Cordeiro, S.L.; Almeida-Lima, J.; Dantas-Santos, N.; Magalhães, K.D.; Sabry, D.A.; Albuquerque, I.R.L.; Pereira, M.R.; Leite, E.L.; et al. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. flabellata. J. Appl. Phycol. 2012, 24, 1159–1167. [Google Scholar] [CrossRef]
- Lin, H.C.; Chou, S.T.; Chuang, M.Y.; Liao, T.Y.; Tsai, W.S.; Chiu, T.H. The effects of Caulerpa microphysa enzyme-digested extracts on ACE-inhibitory activity and in vitro anti-tumour properties. Food Chem. 2012, 134, 2235–2241. [Google Scholar] [CrossRef]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–11. [Google Scholar]
- Osman, O.S.; Selway, J.L.; Harikumar, P.E.; Stocker, C.J.; Wargent, E.T.; Cawthorne, M.A.; Jassim, S.; Langlands, K. A novel method to assess collagen architecture in skin. BMC Bioinform. 2013, 14, 260. [Google Scholar] [CrossRef]
- Ivarsson, M.; McWhirter, A.; Borg, T.K.; Rubin, K. Type I collagen synthesis in cultured human fibroblasts: Regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers. Matrix Biol. 1998, 16, 409–425. [Google Scholar] [CrossRef]
- Risteli, J.; Risteli, L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J. Hepatol. 1995, 22 (Suppl. 2), 77–81. [Google Scholar]
- Risteli, L.; Risteli, J. Non-Invasive Methods for Detection of Organ Fibrosis; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Chang, S.W.; Buehler, M.J. Molecular biomechanics of collagen molecules. Mater. Today 2014, 17, 70–76. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Becher, P.M.; Schulze, K.; Schultheiss, H.P.; Tschöpe, C.; Westermann, D. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem. Res. Int. 2012, 2012, 875742. [Google Scholar] [CrossRef]
- Du, G.; Liu, C.; Li, X.; Chen, W.; He, R.; Wang, X.; Feng, P.; Lan, W. Induction of matrix metalloproteinase-1 by tumor necrosis factor-α is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Exp. Biol. Med. 2016, 241, 2033–2041. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ojima, N.; Yamashita, M. Induction of Mrnas in Response to Acclimation of Trout Cells to Different Osmolalities. Fish Physiol. Biochem. 2000, 22, 255–262. [Google Scholar] [CrossRef]
- Okazaki, M.; Yoshimura, K.; Uchida, G.; Harii, K. Correlation between age and the secretions of melanocyte-stimulating cytokines in cultured keratinocytes and fibroblasts. Br. J. Dermatol. 2005, 153 (Suppl. 2), 23–29. [Google Scholar] [CrossRef]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Ashok, D.; Raghavan, S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front. Cell Dev. Biol. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Rangel, M.A.R.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed Collagen Induces an Anti-Inflammatory Response That Induces Proliferation of Skin Fibroblast and Keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef]
- Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Caulerpenyne from Caulerpa taxifolia has an antiproliferative activity on tumor cell line SK-N-SH and modifies the microtubule network. Life Sci. 2001, 70, 415–429. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.-Y.; Cheng, L.-C.; Hung, Z.-C.; Chen, Z.-Y.; Wang, C.-W.; Hou, H.-H. The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Curr. Issues Mol. Biol. 2024, 46, 2701-2712. https://doi.org/10.3390/cimb46030170
Lu K-Y, Cheng L-C, Hung Z-C, Chen Z-Y, Wang C-W, Hou H-H. The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Current Issues in Molecular Biology. 2024; 46(3):2701-2712. https://doi.org/10.3390/cimb46030170
Chicago/Turabian StyleLu, Kuo-Yun, Li-Ching Cheng, Zheng-Ci Hung, Ze-Ying Chen, Chuang-Wei Wang, and Hsin-Han Hou. 2024. "The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin" Current Issues in Molecular Biology 46, no. 3: 2701-2712. https://doi.org/10.3390/cimb46030170
APA StyleLu, K.-Y., Cheng, L.-C., Hung, Z.-C., Chen, Z.-Y., Wang, C.-W., & Hou, H.-H. (2024). The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Current Issues in Molecular Biology, 46(3), 2701-2712. https://doi.org/10.3390/cimb46030170