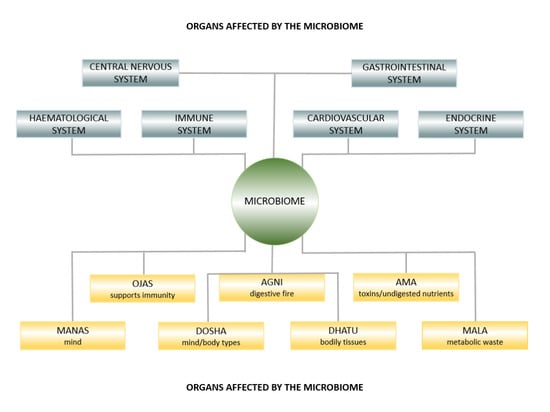
The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda
Abstract
:1. Introduction
2. Ayurveda
3. Prakriti and Gut Bacteria
4. Ayurveda Herbs and Spices and the Microbiome
5. Ama and Leaky Gut Syndrome
6. Biorhythms and Gut Bacteria
7. Probiotic Enemas and Bastis
8. The Gut–Brain Axis and Ojas
9. Stress, Ayurveda, and Psychobiotics
10. The Future of Ayurveda and Modern Medicine
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2019, 9, 2868. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The Role of the Microbiome for Human Health: From Basic Science to Clinical Applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Armour, C.R.; Nayfach, S.; Pollard, K.S.; Sharpton, T.J. A Metagenomic Meta-Analysis Reveals Functional Signatures of Health and Disease in the Human Gut Microbiome. mSystems 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, M.; Li, X.; Parfrey, L.W.; Roth, B.; Ippoliti, A.; Wei, B.; Borneman, J.; Mcgovern, D.P.B.; Frank, D.N.; Li, E.; et al. A Modular Organization of the Human Intestinal Mucosal Microbiota and Its Association with Inflammatory Bowel Disease. PLoS ONE 2013, 8, e80702. [Google Scholar] [CrossRef] [Green Version]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Sommer, F.; Backhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015, 8, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The interplay between the gut microbiota and the immune system. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [Green Version]
- Kozyrskyj, A.L.; Bahreinian, S.; Azad, M.B. Early life exposures: Impact on asthma and allergic disease. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 400–406. [Google Scholar] [CrossRef]
- Burcelin, R. Gut microbiota and immune crosstalk in metabolic disease. Mol. Metab. 2016, 5, 771–781. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Sha, S.; Ni, L.; Stefil, M.; Dixon, M.; Mouraviev, V. The human gastrointestinal microbiota and prostate cancer development and treatment. Investig. Clin. Urol. 2020, 61, S43–S50. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Diet on the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Mendoza, A.E.-H.D.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.W.; Hazen, S.L. Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide and 5-Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE-Like Patient Cohort. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Warrier, M. TrimethylamineN-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Hirata, K.-I. Gut Microbiome and Cardiovascular Diseases. Diseases 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut Microbiota and Cardiovascular Disease: Opportunities and Challenges. Microbiome 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.W.; Bäckhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Sata, Y.; Marques, F.Z.; Kaye, D.M. The Emerging Role of Gut Dysbiosis in Cardio-Metabolic Risk Factors for Heart Failure. Curr. Hypertens. Rep. 2020, 22. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.-Y.; Zhang, Z.; Feng, Y. The Cross-Talk between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef]
- Loverdos, K.; Bellos, G.; Kokolatou, L.; Vasileiadis, I.; Giamarellos, E.; Pecchiari, M.; Koulouris, N.; Koutsoukou, A.; Rovina, N. Lung Microbiome in Asthma: Current Perspectives. J. Clin. Med. 2019, 8, 1967. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.Y.; Chen, Y.X.; Fang, J.Y. 2019 Novel Coronavirus Infection and Gastrointestinal Tract. J. Dig. Dis. 2020, 21, 125–126. [Google Scholar] [CrossRef] [Green Version]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Lyon, L. All disease begins in the gut’: Was Hippocrates right? Brain 2018, 141, e20. [Google Scholar] [CrossRef] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2017, 15, 36–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, T.G.; Cryan, J.F. Melancholic Microbes: A Link between Gut Microbiota and Depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D. Human Gut Microbiome: Hopes, Threats and Promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Barko, P.; Mcmichael, M.; Swanson, K.; Williams, D. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2017, 32, 9–25. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Soares, H.; Raveh-Sadka, T.; Azulay, S.; Edens, K.; Ben-Shlomo, Y.; Cohen, Y.; Ofek, T.; Bachrach, D.; Stevens, J.; Colibaseanu, D.; et al. Assessment of a Personalized Approach to Predicting Postprandial Glycemic Responses to Food Among Individuals Without Diabetes. JAMA Netw. Open 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.B.; Black, A.S.; Sobel, A.L.; Zhao, Y.; Mukherjee, P.; Molparia, B.; Moore, N.E.; Muench, G.R.A.; Wu, J.; Chen, W.; et al. Directed Remodeling of the Mouse Gut Microbiome Inhibits the Development of Atherosclerosis. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Sharma, H. Ayurveda: Science of life, genetics, and epigenetics. AYU 2016, 37, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Gabbianelli, R. Primers on nutrigenetics and nutri(epi)genomics: Origins and development of precision nutrition. Biochimie 2019, 160, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Meade, J.G. Dynamic DNA; SelectBooks: New York, NY, USA, 2018. [Google Scholar]
- Dash, B.; Sharma, R.K. Charaka Samhita; Caukhambha Orientalia: Varanasi, India, 1995. [Google Scholar]
- Prasher, B.; Negi, S.; Aggarwal, S.; Mandal, A.K.; Sethi, T.P.; Deshmukh, S.R.; Purohit, S.G.; Sengupta, S.; Khanna, S.; Mohammad, F.; et al. Whole Genome Expression and Biochemical Correlates of Extreme Constitutional Types Defined in Ayurveda. J. Transl. Med. 2008, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, F.T.; Wallace, R.K. Dosha brain-types: A neural model of individual differences. J. Ayurveda Integr. Med. 2015, 6, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.S.; Pandey, R.; Mondal, A.K.; Gupta, S.; Verma, M.K.; Jain, S.; Ahmed, V.; Patil, R.; Agarwal, D.; Girase, B.; et al. Western Indian Rural Gut Microbial Diversity in Extreme Prakriti Endo-Phenotypes Reveals Signature Microbes. Front. Microbiol. 2018, 9, 118. [Google Scholar] [CrossRef]
- Jnana, A.; Murali, T.S.; Guruprasad, K.P.; Satyamoorthy, K. Prakriti phenotypes as a stratifier of gut microbiome: A new frontier in personalized medicine? [published online ahead of print, 2020 Jul 24]. J. Ayurveda Integr. Med. 2020, S0975-947630041-3. [Google Scholar] [CrossRef]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic Uses of Triphala in Ayurvedic Medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-Free Turmeric Exhibits Anti-Inflammatory and Anticancer Activities: Identification of Novel Components of Turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Mcfadden, R.-M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guruprasad, K.P.; Dash, S.; Shivakumar, M.B.; Shetty, P.R.; Raghu, K.S.; Shamprasad, B.R.; Udupi, V.; Acharya, R.V.; Vidya, P.B.; Nayak, J.; et al. Influence of Amalaki Rasayana on Telomerase Activity and Telomere Length in Human Blood Mononuclear Cells. J. Ayurveda Integr. Med. Med. 2017, 8, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Yadav, C.R.; Meena, M.S. Physiological aspects of Agni. AYU 2010, 31, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H. Leaky gut syndrome, dysbiosis, ama, free radicals, and natural antioxidants. AYU 2009, 30, 88–105. [Google Scholar]
- Fasano, A. Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Sturgeon, C.; Fasano, A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Kecklund, G.; Axelsson, J. Health Consequences of Shift Work and Insufficient Sleep. BMJ 2016, i5210. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, J.L.; Thompson, S.V.; Holscher, H.D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr. Rev. 2017, 75, 673–682. [Google Scholar] [CrossRef]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal Cycling in the Gut Microbiome of the Hadza Hunter-Gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Davenport, E.R.; Mizrahi-Man, O.; Michelini, K.; Barreiro, L.B.; Ober, C.; Gilad, Y. Seasonal Variation in Human Gut Microbiome Composition. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition. Front. Microbiol. 2018, 9, 737. [Google Scholar] [CrossRef]
- Voigt, R.; Forsyth, C.; Green, S.; Engen, P.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 193–205. [Google Scholar] [CrossRef]
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e146643. [Google Scholar] [CrossRef]
- Reynolds, A.C.; Paterson, J.L.; Ferguson, S.A.; Stanley, D.; Wright, K.P.; Dawson, D. The Shift Work and Health Research Agenda: Considering Changes in Gut Microbiota as a Pathway Linking Shift Work, Sleep Loss and Circadian Misalignment, and Metabolic Disease. Sleep Med. Rev. 2017, 34, 3–9. [Google Scholar] [CrossRef]
- Wallace, R.K.; Wallace, S.; Stenberg, S.; Davis, J.; Farley, A. The Rest and Repair Diet; Dharma Publications: Fairfield, IA, USA, 2019. [Google Scholar]
- Perlmutter, D.; Loberg, K. The Brain Maker: The Power of Gut Microbes to Heal and Protect Your Brain for Life; Little Brown and Company: Boston, MA, USA, 2015. [Google Scholar]
- Kadus, P.A.; Vedpathak, S.M. Comparative Study of Anuvasana Basti with Constant and Escalating Dose as an Alternative to Snehapana in Purvakarma of Vamana and Virechana. J. Ayurveda Integr. Med. 2017, 8, 194–199. [Google Scholar] [CrossRef]
- Anu, M.; Kunjibettu, S.; Archana, S.; Dei, L. Management of Premature Contractions with Shatavaryadi Ksheerapaka Basti—A Case Report. AYU 2017, 38, 148. [Google Scholar] [CrossRef]
- Pooja, B.; Bhatted, S. A Standard Controlled Clinical Study on Virechana Karma and Lekhana Basti in the Management of Dyslipidemia (Medoroga). AYU 2016, 37, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadus, P.; Vedpathak, S. Anuvasan Basti in Escalating Dose Is an Alternative for Snehapana before Vamana and Virechana: Trends from a Pilot Study. J. Ayurveda Integr. Med. 2014, 5, 246. [Google Scholar] [CrossRef] [Green Version]
- Shukla, G.; Bhatted, S.; Dave, A.; Shukla, V. Efficacy of Virechana and Basti Karma with Shamana Therapy in the Management of Essential Hypertension: A Comparative Study. AYU 2013, 34, 70. [Google Scholar] [CrossRef] [PubMed]
- Auti, S.; Thakar, A.; Shukla, V.; Ravishankar, B. Assessment of Lekhana Basti in the Management of Hyperlipidemia. AYU 2013, 34, 339. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, S.; Anup, B.; Ashok, B.; Ravishankar, B.; Shukla, V. Evaluation of Anti-Hyperlipidemic Activity of Lekhana Basti in Albino Rats. AYU 2013, 34, 220. [Google Scholar] [CrossRef] [Green Version]
- Baria, R.; Pandya, D.; Joshi, N. Clinical Efficacy of Panchamuladi Kaala Basti (Enema) in the Management of Amavata (Rheumatoid Arthritis). AYU 2011, 32, 90. [Google Scholar] [CrossRef]
- Periasamy, S.; Hsu, D.-Z.; Chandrasekaran, V.R.M.; Liu, M.-Y. Sesame Oil Accelerates Healing of 2,4,6-Trinitrobenzenesulfonic Acid–Induced Acute Colitis by Attenuating Inflammation and Fibrosis. J. Parenter. Enteral. Nutr. 2012, 37, 674–682. [Google Scholar] [CrossRef]
- Hou, R.; Lin, M.; Wang, M.; Tzen, J. Increase of Viability of Entrapped Cells of Lactobacillus Delbrueckii Ssp. Bulgaricus in Artificial Sesame Oil Emulsions. J. Dairy Sci. 2003, 86, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.T.; Lucas, J.; John-Williams, L.S.; Thompson, J.W.; Moseley, M.A.; Patel, S.; Peterson, S.N.; Porter, V.; Schadt, E.E.; Mills, P.J.; et al. Identification of Altered Metabolomic Profiles Following a Panchakarma-Based Ayurvedic Intervention in Healthy Subjects: The Self-Directed Biological Transformation Initiative (SBTI). Sci. Rep. 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Knauf, C. How Gut Microbes Talk to Organs: The Role of Endocrine and Nervous Routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, A.; Åhs, F.; Engman, J.; Jonasson, M.; Alaie, I.; Björkstrand, J.; Frans, Ö.; Faria, V.; Linnman, C.; Appel, L.; et al. Serotonin Synthesis and Reuptake in Social Anxiety Disorder. JAMA Psychiatry 2015, 72, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of Immune Cell Function by Short-Chain Fatty Acids. Clin. Transl. Immunol. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.K. Physiological Effects of Transcendental Meditation. Science 1970, 167, 1751–1754. [Google Scholar] [CrossRef]
- Wallace, R.; Benson, H.; Wilson, A. A Wakeful Hypometabolic Physiologic State. Am. J. Physiol. 1971, 221, 795–799. [Google Scholar] [CrossRef]
- Travis, F.; Shear, J. Focused Attention, Open Monitoring and Automatic Self-Transcending: Categories to Organize Meditations from Vedic, Buddhist and Chinese Traditions. Conscious. Cogn. 2010, 19, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Nidich, S.; Mills, P.J.; Rainforth, M.; Heppner, P.; Schneider, R.H.; Rosenthal, N.E.; Salerno, J.; Gaylord-King, C.; Rutledge, T. Non-Trauma-Focused Meditation versus Exposure Therapy in Veterans with Post-Traumatic Stress Disorder: A Randomised Controlled Trial. Lancet Psychiatry 2018, 5, 975–986. [Google Scholar] [CrossRef]
- Barnes, V.; Orme-Johnson, D. Clinical and Pre-Clinical Applications of the Transcendental Meditation Program® in the Prevention and Treatment of Essential Hypertension and Cardiovascular Disease in Youth and Adults. Curr. Hypertens Rev. 2006, 2, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.H.; Grim, C.E.; Rainforth, M.V.; Kotchen, T.; Nidich, S.I.; Gaylord-King, C.; Salerno, J.W.; Kotchen, J.M.; Alexander, C.N. Stress Reduction in the Secondary Prevention of Cardiovascular Disease. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillisch, K.; Mayer, E.A.; Gupta, A.; Gill, Z.; Brazeilles, R.; Nevé, B.L.; Vlieg, J.E.V.H.; Guyonnet, D.; Derrien, M.; Labus, J.S. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom. Med. 2017, 79, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology 2013, 144. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.K.; Wallace, S. Gut Crisis; Dharma Publications: Fairfield, IA, USA, 2017. [Google Scholar]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, R.K. The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda. Medicina 2020, 56, 462. https://doi.org/10.3390/medicina56090462
Wallace RK. The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda. Medicina. 2020; 56(9):462. https://doi.org/10.3390/medicina56090462
Chicago/Turabian StyleWallace, Robert Keith. 2020. "The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda" Medicina 56, no. 9: 462. https://doi.org/10.3390/medicina56090462
APA StyleWallace, R. K. (2020). The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda. Medicina, 56(9), 462. https://doi.org/10.3390/medicina56090462