Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION
Abstract
:1. Introduction
1.1. New Developments in Polymer and Surface Treatment
1.2. Considerations Regarding TRISKELION
2. Materials and Methods
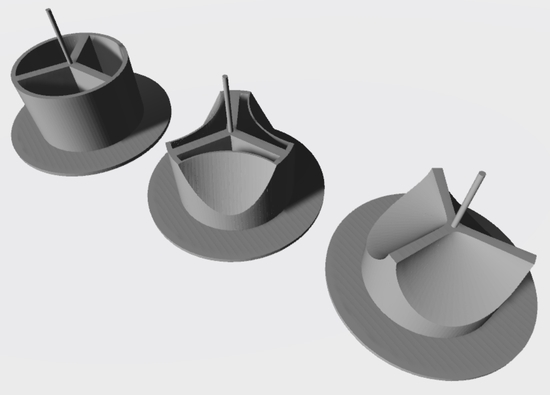
2.1. Design and Construction of Prototypes
2.2. Hemodynamic Measurements and Adaption of the Prototype
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Head, S.J.; Celik, M.; Kappetein, A.P. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Hammermeister, K.E.; Sethi, G.K.; Henderson, W.G.; Oprian, C.; Kim, T.; Rahimtoola, S. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. Veterans Affairs Cooperative Study on Valvular Heart Disease. N. Engl. J. Med. 1993, 328, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Daggett, R.L.; Young, W.P. Development of a carbon-coated, central-hinging, bileaflet valve. Ann. Thorac. Surg. 1989, 48, S28–S30. [Google Scholar] [CrossRef]
- DeWall, R.A.; Qasim, N.; Carr, L. Evolution of mechanical heart valves. Ann. Thorac. Surg. 2000, 69, 1612–1621. [Google Scholar] [CrossRef]
- Persson, M.; Glaser, N.; Franco-Cereceda, A.; Nilsson, J.; Holzmann, M.J.; Sartipy, U. Porcine vs Bovine Bioprosthetic Aortic Valves: Long-Term Clinical Results. Ann. Thorac. Surg. 2021, 111, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Alejo, D.E.; Cameron, D.E. Mechanical heart valves: 50 years of evolution. Ann. Thorac. Surg. 2003, 76, S2230–S2239. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, S.L.; Ferrans, V.J.; Tomita, Y.; Eidbo, E.E.; Jones, M. Evaluation of explanted polyurethane trileaflet cardiac valve prostheses. J. Thorac. Cardiovasc. Surg. 1987, 94, 419–429. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Ghanavi, J.E.; Narula, A.; Odlyha, M.; Peirovi, H.; Butler, P.E.; Seifalian, A.M. Silsesquioxane nanocomposites as tissue implants. Plast. Reconstr. Surg. 2007, 119, 1653–1662. [Google Scholar] [CrossRef]
- de Mel, A.; Punshon, G.; Ramesh, B.; Sarkar, S.; Darbyshire, A.; Hamilton, G.; Seifalian, A.M. In situ endothelialization potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graft. Biomed. Mater. Eng. 2009, 19, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; De Groot, J.; Clatworthy, I.; Bozec, L.; Horton, M.; Butler, P.E.; Seifalian, A.M. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules 2006, 7, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; Yap, J.; Seifalian, A.M.; Burriesci, G. A new transcatheter heart valve concept (the TRISKELE): Feasibility in an acute preclinical model. EuroIntervention 2016, 12, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Langanki, D.; Ogle, M.F.; Cameron, J.D.; Lirtzman, R.A.; Schroeder, R.F.; Mirsch, M.W. Evaluation of a novel bioprosthetic heart valve incorporating anticalcification and antimicrobial technology in a sheep model. J. Heart Valve Dis. 1998, 7, 633–638. [Google Scholar]
- Ghanbari, H.; de Mel, A.; Seifalian, A.M. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Int. J. Nanomed. 2011, 6, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Corden, J.; David, T.; Fisher, J. Determination of the curvatures and bending strains in open trileaflet heart valves. Proc. Inst. Mech. Eng. H 1995, 209, 121–128. [Google Scholar] [CrossRef]
- Schroter, F.; Hartrumpf, M.; Kuehnel, R.U.; Ostovar, R.; Albes, J.M. Further Evolution of a New Nonbiological Transcatheter Valvular Prosthesis. Thorac. Cardiovasc. Surg. 2021, 69, 43–48. [Google Scholar] [CrossRef]
- Kuehnel, R.U.; Pohl, A.; Puchner, R.; Wendt, M.O.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Opening and closure characteristics of different types of stented biological valves. Thorac. Cardiovasc. Surg. 2006, 54, 85–90. [Google Scholar] [CrossRef]
- Kuehnel, R.U.; Puchner, R.; Pohl, A.; Wendt, M.O.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Characteristic resistance curves of aortic valve substitutes facilitate individualized decision for a particular type. Eur. J. Cardiothorac. Surg. 2005, 27, 450–455, discussion 455. [Google Scholar] [CrossRef]
- Kuehnel, R.U.; Wendt, M.O.; Jainski, U.; Hartrumpf, M.; Pohl, M.; Albes, J.M. Suboptimal geometrical implantation of biological aortic valves provokes functional deficits. Interact. Cardiovasc. Thorac. Surg. 2010, 10, 971–975, discussion 975. [Google Scholar] [CrossRef] [Green Version]
- Schichl, K.; Affeld, K. A computer controlled versatile pulse duplicator for precision testing of artificial heart valves. Int. J. Artif. Organs 1993, 16, 722–728. [Google Scholar] [CrossRef] [PubMed]





| A1 | B2 | C2 | C2.1 | C3.1.1 | C4.1.3 | C5 | Peri- Mount | Medtronic Advantage | |
|---|---|---|---|---|---|---|---|---|---|
| Closing time (ms) | 118.00 ± 15.94 | 103.67 ± 2.87 | 97.58 ± 8.16 | 61.92 ± 4.07 | 72.67 ± 6.07 | 81.73 ± 5.41 | 86.96 ± 13.40 | 42.33 ± 7.15 | 40.00 ± 7.03 |
| Closing volume (mL) | 20.15 ± 2.70 | 17.60 ± 1.22 | 12.96 ± 1.55 | 6.66 ± 0.46 | 7.92 ± 0.51 | 9.70 ± 0.63 | 10.37 ± 2.03 | 2.78 ± 0.26 | 2.87 ± 0.49 |
| Leak volume (mL) | 35.21 ± 1.97 | 31.58 ± 2.28 | 11.70 ± 8.50 | 19.34 ± 2.17 | 12.78 ± 6.75 | 10.21 ± 7.92 | 5.16 ± 3.35 | 3.18 ± 0.34 | 6.67 ± 0.57 |
| Cardiac output (L/min) | 1.01 ± 0.08 | 1.44 ± 0.21 | 3.15 ± 0.65 | 3.06 ± 0.15 | 3.44 ± 0.50 | 3.48 ± 0.59 | 3.80 ± 0.21 | 4.46 ± 0.01 | 4.21 ± 0.05 |
| Regurgitation fraction (%) | 79.34 ± 1.68 | 70.52 ± 4.42 | 35.36 ± 13.24 | 37.25 ± 3.13 | 29.62 ± 10.24 | 28.54 ± 12.11 | 22.26 ± 4.34 | 8.55 ± 0.22 | 13.23 ± 0.79 |
| Systolic pressure gradient (mmHg) | 8.60 ± 0.13 | 11.77 ± 0.50 | 11.17 ± 3.57 | 11.97 ± 0.89 | 9.87 ± 0.40 | 8.03 ± 0.53 | 9.93 ± 3.22 | 8.18 ± 0.65 | 10.15 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschorn, P.; Schröter, F.; Hartrumpf, M.; Kühnel, R.-U.; Ostovar, R.; Albes, J.M. Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION. Medicina 2022, 58, 1695. https://doi.org/10.3390/medicina58111695
Tschorn P, Schröter F, Hartrumpf M, Kühnel R-U, Ostovar R, Albes JM. Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION. Medicina. 2022; 58(11):1695. https://doi.org/10.3390/medicina58111695
Chicago/Turabian StyleTschorn, Philip, Filip Schröter, Martin Hartrumpf, Ralf-Uwe Kühnel, Roya Ostovar, and Johannes M. Albes. 2022. "Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION" Medicina 58, no. 11: 1695. https://doi.org/10.3390/medicina58111695
APA StyleTschorn, P., Schröter, F., Hartrumpf, M., Kühnel, R. -U., Ostovar, R., & Albes, J. M. (2022). Engineering a New Polymeric Heart Valve Using 3D Printing—TRISKELION. Medicina, 58(11), 1695. https://doi.org/10.3390/medicina58111695