Sesquiterpene and Acetogenin Derivatives from the Marine Red Alga Laurencia okamurai
Abstract
:1. Introduction

2. Results and Discussion
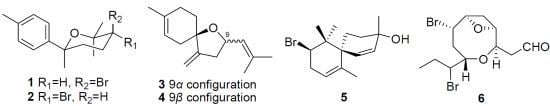
Structure Elucidation of the New Compounds
| No. | 1 (CDCl3) | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1/5 | 7.34, d (8.0) | 124.8, CH | 7.34, d (8.1) | 126.0, CH |
| 2/4 | 7.11, d (8.0) | 128.7, CH | 7.12, d (8.1) | 128.6, CH |
| 3 | 136.1, C | 136.4, C | ||
| 6 | 146.0, C | 143.2, C | ||
| 7 | 74.6, C | 74.4, C | ||
| 8eq | 2.16, m | 34.1, CH2 | 2.56, m | 36.0, CH2 |
| 8ax | 2.10, m | 2.18, m | ||
| 9eq | 2.28, m | 28.2, CH2 | 2.27, m | 29.4, CH2 |
| 9ax | 2.25, m | 1.82, m | ||
| 10 | 4.05, dd (7.9, 4.4) | 59.1, CH | 4.04, dd (12.1, 4.1) | 59.0, CH |
| 11 | 75.2, C | 76.4, C | ||
| 12 | 1.47, s | 27.8, CH3 | 1.35, s | 22.5, CH3 |
| 13 | 1.14, s | 29.4, CH3 | 0.78, s | 30.8, CH3 |
| 14 | 1.50, s | 31.8, CH3 | 1.36, s | 35.8, CH3 |
| 15 | 2.23, s | 20.9, CH3 | 2.34, s | 21.0, CH3 |


| No. | 3 | 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 2.25, m | 38.1, CH2 | 2.18, m | 36.8, CH2 | 5.54, d | 131.2, CH | 9.80, br s | 199.3, CH |
| (10.4) | ||||||||
| 2a | 5.34, m | 119.1, CH | 5.34, m | 119.0, CH | 5.85, d | 136.5, CH | 2.67, dd | 42.4, CH2 |
| (10.4) | (17.5, 6.2) | |||||||
| 2b | 3.06, dd | |||||||
| (17.3, 7.9) | ||||||||
| 3 | 133.4, C | 133.7, C | 67.4, C | 4.34, t (6.5) | 72.7, CH | |||
| 4a | 1.93, m | 27.7, CH2 | 1.93, m | 28.0, CH2 | 1.56, m | 28.5, CH2 | 4.65, dd | 81.6, CH |
| (8.7, 5.0) | ||||||||
| 4b | 2.22, m | 2.22, m | 1.99, m | |||||
| 5a | 1.58, m | 31.5, CH2 | 1.66, m | 34.5, CH2 | 1.78, m | 36.3, CH2 | 2.75, m | 21.7, CH2 |
| 5b | 1.82, m | 1.75, m | 2.91, m | |||||
| 6 | 80.9, C | 80.8, C | 47.4, C | 4.97, m | 80.9, CH | |||
| 7 | 156.3, C | 156.5, C | 139.5, C | 4.21, m | 50.4, CH | |||
| 8a | 2.38, m | 40.1, CH2 | 2.38, m | 40.3, CH2 | 5.23, m | 120.8, CH | 2.42, dd | 41.7, CH2 |
| (14.1, 5.8) | ||||||||
| 8b | 2.71, dd (15.7, 9.7) | 2.61, dd (15.6, 9.5) | 2.61, m | |||||
| 9 | 4.63, m | 71.8, CH | 4.63, m | 72.8, CH | 2.58, m | 36.1, CH2 | 4.50, dd | 74.4, CH |
| (7.4, 3.5) | ||||||||
| 10 | 5.22, m | 126.0, CH | 5.22, m | 126.2, CH | 4.64, dd | 61.4, CH | 3.80, dt | 64.1, CH |
| (10.6, 6.4) | (11.5, 3.5) | |||||||
| 11a | 136.2, C | 135.5, C | 41.6, C | 1.77, m | 27.4, CH2 | |||
| 11b | 1.88, m | |||||||
| 12 | 1.69, s | 18.2, CH3 | 1.70, s | 18.3, CH3 | 1.02, s | 18.1, CH3 | 1.07, t (7.7) | 12.8, CH3 |
| 13 | 1.71, s | 25.8, CH3 | 1.71, s | 25.8, CH3 | 1.11, s | 26.3, CH3 | ||
| 14a | 4.78, br s | 103.5, CH2 | 4.78, br s | 103.8, CH2 | 1.57, s | 21.9, CH3 | ||
| 14b | 4.90, br s | 4.91, br s | ||||||
| 15 | 1.66, s | 23.4, CH3 | 1.66, s | 23.4, CH3 | 1.31, s | 28.8, CH3 | ||
3. Experimental Section
3.1. General
3.2. Algal Material
3.3. Extraction and Isolation
3.4. Computational Details
3.5. Brine Shrimp Toxicity
4. Conclusions
Acknowledgments
References
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Zhang, Y.; Wang, B.-G. Two new halogenated chamigrane-type sesquiterpenes and other secondary metabolites from the marine red alga Laurencia okamurai and their chemotaxonomic significance. Biochem. Syst. Ecol. 2007, 35, 627–630. [Google Scholar] [CrossRef]
- Sugimoto, M.; Suzuki, T.; Hagiwara, H.; Hoshi, T. The first total synthesis of (+)-(Z)-laureatin. Tetrahedron Lett. 2007, 48, 1109–1112. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1996, 13, 75–125. [Google Scholar]
- Masuda, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and chemotaxonomic studies on Laurencia composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar] [CrossRef]
- Carvalho, L.R.; Fujii, M.T.; Roque, N.F.; Lago, H.G. Aldingenin derivatives from the red alga Laurencia aldingensis. Phytochemistry 2006, 67, 1331–1335. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Li, K.; Wang, B.-G. Laurendecumallenes A–B and laurendecumenynes A–B, halogenated nonterpenoid C15-acetogenins from the marine red alga Laurencia decumbens. J. Nat. Prod. 2007, 70, 1499–1502. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Ding, L.-P.; Wang, B.-G. Diterpenes, sesquiterpenes, and a C15-acetogenin from the marine red alga Laurencia mariannensis. J. Nat. Prod. 2007, 70, 1901–1905. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Ding, L.-P.; Wang, B.-G. Aristolane sesquiterpenes and highly brominated indoles from the marine red alga Laurencia similis (Rhodomelaceae). Helv.Chim.Acta 2007, 90, 385–391. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.M.; Cui, C.M.; Li, C.S.; Wang, B.G. A new rearranged chamigrane sesquiterpene from Laurencia okamurai. Chin. Chem. Lett. 2009, 20, 190–192. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Li, K.; Wang, B.-G. Halogenated sesquiterpenes from the marine red alga Laurencia saitoi (Rhodomelaceae). Helv. Chim. Acta 2009, 92, 1873–1879. [Google Scholar] [CrossRef]
- Li, X.-D.; Miao, F.-P.; Li, K.; Ji, N.-Y. Sesquiterpenes and acetogenins from the marine red alga Laurencia okamurai. Fitoterapia 2012, 83, 518–522. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E. (3E)-Laureatin and (3E)-isolaureatin, halogenated C-15 non-terpenoid compounds from the red alga Laurencia nipponica Yamada. Bull. Chem. Soc. Jpn. 1987, 60, 3791–3792. [Google Scholar] [CrossRef]
- Wright, A.D.; König, G.M.; De Nys, R.; Sticher, O. Seven new metabolites from the marine red alga Laurencia majuscula. J. Nat. Prod. 1993, 56, 394–401. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J. Metabolites of the red alga Laurencia subopposita. J. Org. Chem. 1977, 42, 3343–3349. [Google Scholar] [CrossRef]
- Irie, T.; Suzuki, T.; Yasunari, Y.; Kurosawa, E. Laurene, a sesquiterpene hydrocarbon from Laurencia species. Tetrahedron 1969, 25, 459–468. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. New laurene derivatives from Laurencia filiformis. Aust. J. Chem. 1976, 29, 2533–2539. [Google Scholar] [CrossRef]
- Nemoto, H.; Miyata, J.; Hakamata, H.; Nagamochi, M.; Fukumoto, K. A novel and efficient route to chiral A-ring aromatic trichothecanes—The first enantiocontrolled total synthesis of (−)-debromofiliformin and (−)-filiformin. Tetrahedron 1995, 51, 5511–5522. [Google Scholar]
- Suzuki, M.; Kurosawa, E. Halogenated sesquiterpene phenols and ethers from the red alga Laurencia glandulifera Kutzing. Bull. Chem. Soc. Jpn. 1979, 52, 3349–3351. [Google Scholar] [CrossRef]
- De Nys, R.; Coll, J.C.; Bowden, B.F. Tropical marine algae. IX. A new sesquiterpenoid metabolite from the red alga Laurencia rnarianensis. Aust. J. Chem. 1993, 46, 933–937. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E.; Furusaki, A. The structure and absolute stereochemistry of a Halogenated chamigrene derivative from the red alga Laurencia species. Bull. Chem. Soc. Jpn. 1988, 61, 3371–3373. [Google Scholar] [CrossRef]
- Fukuzawa, A.; Honma, T.; Takasugi, Y.; Murai, A. Biogenetic intermediates, (3E and 3Z,12Z)-laurediols and (3E and 3Z)-12,13-dihydrolaurediols, isolated from Laurencia nipponica. Phytochemistry 1993, 32, 1435–1438. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E.; Furusaki, A.; Katsuragi, S. Neolaurallene, a new halogenated C-15 nonterpenoid from the red alga Laurencia okamurai Yamada. Chem. Lett. 1984, 1033–1034. [Google Scholar]
- Aydoğmuş, Z.; Imre, S.; Ersoy, L.; Wray, V. Halogenated secondary metabolites from Laurencia obtusa. Nat. Prod. Res. 2004, 18, 43–49. [Google Scholar] [CrossRef]
- Irie, T.; Izawa, M.; Kurosawa, E. Laureatin, a constituent from Laurencia nipponica Yamada. Tetrahedron Lett. 1968, 24, 2091–2096. [Google Scholar]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Hamel, E.; Blokhin, A.; Slate, D. Structure of curacin A, a novel antimitotic, antiproliferative, and brine shrimp toxic natural product from the marine Cyanobacterium Lyngbya majuscule. J. Org. Chem. 1994, 59, 1243–1245. [Google Scholar]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, Y.; Li, X.-M.; Cui, C.-M.; Li, C.-S.; Sun, H.; Wang, B.-G. Sesquiterpene and Acetogenin Derivatives from the Marine Red Alga Laurencia okamurai. Mar. Drugs 2012, 10, 2817-2825. https://doi.org/10.3390/md10122817
Liang Y, Li X-M, Cui C-M, Li C-S, Sun H, Wang B-G. Sesquiterpene and Acetogenin Derivatives from the Marine Red Alga Laurencia okamurai. Marine Drugs. 2012; 10(12):2817-2825. https://doi.org/10.3390/md10122817
Chicago/Turabian StyleLiang, Yi, Xiao-Ming Li, Chuan-Ming Cui, Chun-Shun Li, Hong Sun, and Bin-Gui Wang. 2012. "Sesquiterpene and Acetogenin Derivatives from the Marine Red Alga Laurencia okamurai" Marine Drugs 10, no. 12: 2817-2825. https://doi.org/10.3390/md10122817
APA StyleLiang, Y., Li, X. -M., Cui, C. -M., Li, C. -S., Sun, H., & Wang, B. -G. (2012). Sesquiterpene and Acetogenin Derivatives from the Marine Red Alga Laurencia okamurai. Marine Drugs, 10(12), 2817-2825. https://doi.org/10.3390/md10122817