A New Antiproliferative and Antioxidant Peptide Isolated from Arca subcrenata
Abstract
:1. Introduction
2. Results
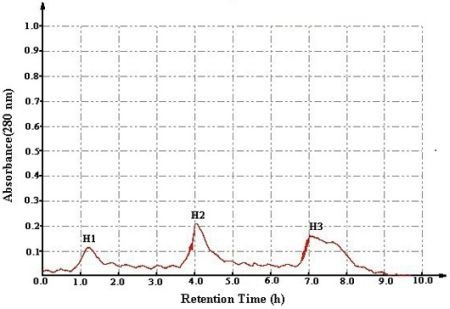
2.1. Bioassay-Guided Isolation in Vitro
| Samples | IC50 (μg/mL) | ||||
|---|---|---|---|---|---|
| HeLa | A549 | HepG2 | HT-29 | SPC-A-1 | |
| Total proteins | 106.5 ± 13.2 | 836.2 ± 76.3 | 264.8 ± 20.6 | 315.4 ± 25.3 | 635.8 ± 46.5 |
| Fraction I | 305.3 ± 24.1 | 1089.6 ± 85.4 | 316.8 ± 20.9 | 325.7 ± 19.8 | 587.4 ± 30.6 |
| Fraction II | 265.5 ± 17.2 | 813.7 ± 80.4 | 274.3 ± 22.6 | 295.9 ± 18.1 | 567.5 ± 34.3 |
| Fraction III | 85.3 ± 7.3 | 789.4 ± 75.6 | 83.7 ± 5.6 | 92.5 ± 8.2 | 314.4 ± 28.5 |
| Fraction P1 | 312.6 ± 26.8 | 632.5 ± 83.1 | 214.5 ± 11.4 | 255.4 ± 14.8 | 468.2 ± 28.6 |
| Fraction P2 | 11.4 ± 3.1 | 232.2 ± 14.6 | 10.4 ± 2.7 | 13.0 ± 3.3 | 727.6 ± 67.9 |
| Fraction P3 | 231.7 ± 14.3 | 547.4 ± 27.8 | 275.4 ± 15.6 | 258.5 ± 21.5 | 889.2 ± 86.7 |
| Fraction P4 | 327.2 ± 19.1 | 778.6 ± 70.8 | 253.8 ± 12.6 | 308.2 ± 25.4 | 845.3 ± 83.9 |
| H1 | 146.7 ± 13.6 | nd | 132.2 ± 11.5 | 135.3 ± 12.5 | nd |
| H2 | 133.6 ± 12.3 | nd | 120.7 ± 10.8 | 125.7 ± 11.3 | nd |
| H3 | 10.8 ± 2.8 | nd | 10.1 ± 2.3 | 10.5 ± 2.4 | nd |
| Cisplatin | 1.13 ± 0.06 | 0.87 ± 0.03 | 0.98 ± 0.04 | 0.94 ± 0.04 | 2.32 ± 0.07 |


2.2. Characterization of Purified Peptide



2.3. De Novo Sequencing of Peptide
2.4. In Vitro Antioxidant Activity

Discussion
4. Experimental Section
4.1. Materials
4.2. Sample Collection
4.3. Preparation of Crude Protein
4.4. In Vitro Antiproliferative Assay
4.4.1. Cell Lines and Culture Conditions
4.4.2. Cell Antiproliferative Assay
4.4.3. Cytotoxicity Assay
4.5. Purification of Antiproliferative Peptide
4.5.1. Anion Exchange Chromatography
4.5.2. Hydrophobic Chromatography
4.6. Characterization of Antiproliferative Peptide
4.6.1. Protein Determination
4.6.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6.3. Isoelectric Focusing-Polyacrylamide Gel Electrophoresis (IEF)
4.6.4. Reversed Phase-High Performance Liquid Chromatography (RP-HPLC)
4.6.5. Molecular Mass Determination
4.7. Identification of Peptide by MALDI-TOF/TOF-MS
4.8. Determination of in Vitro Antioxidant Activities
4.8.1. DPPH Radical Scavenging Activity
4.8.2. Hydroxyl Radical Scavenging Activity
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Willmott, L.J.; Monk, B.J. Cervical cancer therapy: Current, future and anti-angiogensis targeted treatment. Expert Rev. Anticancer Ther. 2009, 9, 895–903. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Liu, R.H.; Regenstein, J.M. Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J. Agric. Food Chem. 2011, 59, 7948–7953. [Google Scholar] [CrossRef]
- Griffin, C.; Karnik, A.; McNulty, J.; Pandey, S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol. Cancer Ther. 2011, 10, 57–68. [Google Scholar]
- Danese, E.; Montagnana, M.; Minicozzi, A.M.; de Matteis, G.; Scudo, G.; Salvagno, G.L.; Cordiano, C.; Lippi, G.; Guidi, G.C. Real-time polymerase chain reaction quantification of free DNA in serum of patients with polyps and colorectal cancers. Clin. Chem. Lab. Med. 2010, 48, 1665–1668. [Google Scholar]
- Beaglehole, R.; Bonita, R.; Magnusson, R. Global cancer prevention: An important pathway to global health and development. Public Health 2011, 125, 821–831. [Google Scholar] [CrossRef]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.J. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Margolin, K.; Longmate, J.; Synold, T.W.; Gandara, D.R.; Weber, J.; Gonzalez, R.; Johansen, M.J.; Newman, R.; Baratta, T.; Doroshow, J.H. Dolastatin-10 in metastatic melanoma: A phase II and pharmokinetic trial of the California Cancer Consortium. Invest. New Drugs 2001, 19, 335–340. [Google Scholar] [CrossRef]
- Suarez, Y.; Gonzalez, L.; Cuadrado, A.; Berciano, M.; Lafarga, M.; Munoz, A.; Kahalalide, F. A new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol. Cancer Ther. 2003, 2, 863–872. [Google Scholar]
- Holzinger, A.; Meindl, U. Jasplakinolide, a novel actin targeting peptide, inhibits cell growth and induces actin filament polymerization in the green alga Micrasterias. Cell Motil. Cytoskeleton 1997, 38, 365–372. [Google Scholar] [CrossRef]
- Ueoka, R.; Ise, Y.; Ohtsuka, S.; Okada, S.; Yamori, T.; Matsunaga, S. Yaku’amides A and B, cytotoxic linear peptides rich in dehydroamino acids from the marine sponge Ceratopsion sp. J. Am. Chem. Soc. 2010, 132, 17692–17694. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, Y. Marine Chinese Materia Medica; Science Press: Beijing, China, 2003; pp. 138–141. [Google Scholar]
- Li, Q.; Li, T.M.; Wang, X.Q.; Huang, Q.; Wu, W.J. Biochemical properties analysis of Arca subcrenata extractive. Pharm. Biotechnol. 1988, 5, 245–247. [Google Scholar]
- Dou, C.G.; Yan, Y.Q.; Zhang, Z. Experimental studies on hypoglycemia and hypolipid effects of hydrolysate of Arca subcrenata. Chin. J. Mar. Drugs 1996, 15, 13–15. [Google Scholar]
- Song, L.Y.; Ren, S.F.; Yu, R.M.; Yan, C.Y.; Li, T.F.; Zhao, Y. Purification, characterization and in vitro anti-tumor activity of proteins from Arca subcrenata Lischke. Mar. Drugs 2008, 6, 418–430. [Google Scholar] [CrossRef]
- Song, L.Y.; Li, T.F.; Yu, R.M.; Yan, C.Y.; Ren, S.F.; Zhao, Y. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Mar. Drugs 2008, 6, 607–619. [Google Scholar] [CrossRef]
- Lazcano-Perez, F.; Roman-Gonzalez, S.A.; Sanchez-Puig, N.; Arreguin-Espinosa, R. Bioactive peptides from marine organisms: A short overview. Protein Pept. Lett. 2012, 19, 700–707. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Wang, C.G.; Liu, H.Z.; Wang, F.X.; Zheng, L.H.; Zhao, J.; Chu, E.; Lin, X.K. A novel polypeptide extracted from Ciona savignyi induces aapoptosis through a mitochondrial-mediated pathway in human colorectal carcinoma cells. Clin. Colorectal Cancer 2012, 11, 207–214. [Google Scholar] [CrossRef]
- Guo, X.N.; Zhu, K.X.; Zhang, H.; Yao, H.Y. Purification and characterization of the antitumor protein from Chinese tartary buckwheat (Fagopyrum tataricum Gaertn) water-soluble extracts. J. Agric. Food Chem. 2007, 55, 6958–6961. [Google Scholar] [CrossRef]
- Naqash, S.Y.; Nazeer, R.A. In vitro antioxidant and antiproliferative activities of bioactive peptide isolated from Nemipterus Japonicus Backbone. Int. J. Food Prop. 2012, 15, 1200–1211. [Google Scholar] [CrossRef]
- Gao, X.; Lu, X.F.; Li, S.F.; Gao, K.; Cheng, J.Q. Antibacterial activity and cytotoxicity of polypeptide dhvar4. Sichuan Da Xue Xue Bao Yi Xue Ban 2005, 36, 308–311. [Google Scholar]
- Wang, H.H.; Xiao, J.W.; Li, B.; Zhong, C.G. Protective effect of reduced glutathione on cytotoxicity induced by hexavalent chromium [Cr(VI)] in L-02 hepatocyte. Wei Sheng Yan Jiu 2006, 35, 414–415. [Google Scholar]
- Wang, M.; Nie, Y.; Peng, Y.; He, F.; Yang, J.; Wu, C.; Li, X. Purification, characterization and antitumor activities of a new protein from Syngnathus acus, an officinal marine fish. Mar. Drugs 2011, 10, 35–50. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2011, 42, 1641–1649. [Google Scholar] [CrossRef]
- Butterfield, D.; Castegna, A.; Pocernich, C.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444–447. [Google Scholar] [CrossRef]
- Pryor, W.A. Free radical biology: Xenobiotics, cancer, and aging. Ann. N. Y. Acad. Sci. 1982, 393, 1–22. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.; Johnson, M.G.; Nannapaneni, R. Human colon and liver cancer cell proliferation inhibition by peptide hydrolysates derived from heat-stabilized defatted rice bran. J. Agric. Food Chem. 2008, 56, 11643–11647. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Simonsen, N.; Arab, L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer-related mortality among postmenopausal women. Nutr. Cancer 2003, 46, 15–22. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Chinery, R.; Brockman, J.A.; Peeler, M.O.; Shyr, Y.; Beauchamp, R.D.; Coffey, R.J. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: A p53-independent induction of p21WAF1/CIP1 via C/EBPbeta. Nat. Med. 1997, 3, 1233–1241. [Google Scholar] [CrossRef]
- Murphy, E.J.; Edmondson, R.D.; Russell, D.H.; Colles, S.; Schroeder, F. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim. Biophys. Acta 1436, 413–425. [Google Scholar]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Stephano, J.L.; Gould, M.; Rojas-Galicia, L. Advantages of picrate fixation for staining polypeptides in polyacrylamide gels. Anal. Biochem. 1986, 152, 308–313. [Google Scholar] [CrossRef]
- Righetti, P.G.; Gianazza, E. Isoelectric focusing in immobilized pH gradients: Theory and newer methodology. Methods Biochem. Anal. 1987, 32, 215–278. [Google Scholar] [CrossRef]
- Guedes, S.D.M.; Vitorino, R.; Tomer, K.; Domingues, M.R.M.; Correia, A.J.F.; Amado, F.; Domingues, P. Drosophila melanogaster larval hemolymph protein mapping. Biochem. Biophys. Res. Commun. 2003, 312, 545–554. [Google Scholar] [CrossRef]
- Yergey, A.L.; Coorssen, J.B.; Backund, P.S.; Humphrep, G.A.; Zimmerberg, J. De novo sequencing of peptides using MALDI/TOF-TOF. J. Am. Soc. Mass Spectrum. 2002, 13, 784–791. [Google Scholar] [CrossRef]
- Hu, X.Q.; Huang, Y.Y.; Dong, Q.F.; Song, L.Y.; Yuan, F.; Yu, R.M. Structure characterization and antioxidant activity of a novel polysaccharide isolated from pulp tissues of Litchi chinensis. J. Agric. Food Chem. 2011, 59, 11548–11552. [Google Scholar]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, L.; Song, L.; Li, T.; Zhu, J.; Xu, J.; Zheng, Q.; Yu, R. A New Antiproliferative and Antioxidant Peptide Isolated from Arca subcrenata. Mar. Drugs 2013, 11, 1800-1814. https://doi.org/10.3390/md11061800
Chen L, Song L, Li T, Zhu J, Xu J, Zheng Q, Yu R. A New Antiproliferative and Antioxidant Peptide Isolated from Arca subcrenata. Marine Drugs. 2013; 11(6):1800-1814. https://doi.org/10.3390/md11061800
Chicago/Turabian StyleChen, Lili, Liyan Song, Tingfei Li, Jianhua Zhu, Jian Xu, Qin Zheng, and Rongmin Yu. 2013. "A New Antiproliferative and Antioxidant Peptide Isolated from Arca subcrenata" Marine Drugs 11, no. 6: 1800-1814. https://doi.org/10.3390/md11061800
APA StyleChen, L., Song, L., Li, T., Zhu, J., Xu, J., Zheng, Q., & Yu, R. (2013). A New Antiproliferative and Antioxidant Peptide Isolated from Arca subcrenata. Marine Drugs, 11(6), 1800-1814. https://doi.org/10.3390/md11061800