Effect of Astaxanthin on Human Sperm Capacitation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Asta on Sperm Endogenous ROS Generation

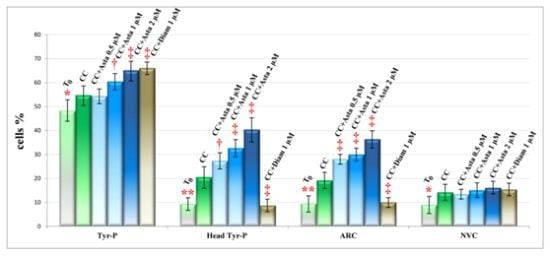
2.2. Effect of Asta on Sperm Tyr-P Pattern

2.3. Effect of Asta on Sperm Viability and Acrosome Reaction (AR)


3. Experimental Section
3.1. Semen Collection and Analysis
3.2. Chemicals
3.3. Sample Preparation
3.4. ROS Enhanced Chemiluminescence (ECL)
3.5. Anti-P-Tyr Evaluation at Confocal Microscopy
3.6. Evaluation of Acrosome Reaction
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Olds-Clarke, P. Unresolved issues in mammalian fertilization. Int. Rev. Cytol. 2003, 232, 129–184. [Google Scholar] [CrossRef]
- Liguori, L.; de Lamirande, E.; Minelli, A.; Gagnon, C. Various protein kinases regulate human sperm acrosome reaction and the associated phosphorylation of Tyr residues and of the Thr-Glu-Tyr motif. Mol. Hum. Reprod. 2005, 11, 211–221. [Google Scholar] [CrossRef]
- De Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 1998, 111, 645–656. [Google Scholar]
- De Lamirande, E.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human sperm. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J., Jr.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999, 14, 2801–2807. [Google Scholar] [CrossRef]
- Moustafa, M.H.; Sharma, R.K.; Thornton, J.; Mascha, E.; Abdel-Hafez, M.A.; Thomas, A.J., Jr.; Agarwal, A. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum. Reprod. 2004, 19, 129–138. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Pasqualotto, F.F.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil. Steril. 2000, 73, 459–464. [Google Scholar] [CrossRef]
- Donà, G.; Fiore, C.; Tibaldi, E.; Frezzato, F.; Andrisani, A.; Ambrosini, G.; Fiorentin, D.; Armanini, D.; Bordin, L.; Clari, G. Endogenous reactive oxygen species content and modulation of tyrosine phosphorylation during sperm capacitation. Int. J. Androl. 2011, 34, 411–419. [Google Scholar] [CrossRef]
- Donà, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Brunati, A.M.; Ragazzi, E.; Armanini, D.; Bordin, L.; Clari, G. Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum. Reprod. 2011, 26, 3264–3273. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S.; Kurimura, Y.; Tsuji, Y. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 1997, 48, 351–356. [Google Scholar] [CrossRef]
- Terao, J. Antioxidant activity of β-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 1999, 70, 185–189. [Google Scholar]
- Comhaire, F.H.; El Garem, Y.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar]
- Bordin, L.; Ion-Popa, F.; Brunati, A.M.; Clari, G.; Low, P.S. Effector-induced Syk-mediated phosphorylation in human erythrocytes. Biochim. Biophys. Acta 2005, 1745, 20–28. [Google Scholar] [CrossRef]
- Nixon, B.; Mitchell, L.A.; Anderson, A.L.; McLaughlin, E.A.; O’bryan, M.K.; Aitken, R.J. Proteomic and functional analysis of human sperm detergent resistant membranes. J. Cell. Physiol. 2011, 226, 2651–2665. [Google Scholar] [CrossRef]
- Choi, Y.H.; Toyoda, Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 1998, 59, 1328–1333. [Google Scholar] [CrossRef]
- Botto, L.; Bernabò, N.; Palestini, P.; Barboni, B. Bicarbonate induces membrane reorganization and CBR1 and TRPV1 endocannabinoid receptor migration in lipid microdomains in capacitating boar spermatozoa. J. Membr. Biol. 2010, 238, 33–41. [Google Scholar] [CrossRef]
- Sleight, S.B.; Miranda, P.V.; Plaskett, N.W.; Maier, B.; Lysiak, J.; Scrable, H.; Herr, J.C.; Visconti, P.E. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: Evidence for dissociation of lipid rafts during capacitation. Biol. Reprod. 2005, 73, 721–729. [Google Scholar] [CrossRef]
- World Health Organization, WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th ed; Cambridge University Press: Cambridge, UK, 1999; pp. 96–99.
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Aitken, R.J.; Buckingham, D.W.; West, K.M. Reactive oxygen species and human spermatozoa analysis of the cellular mechanisms involved in luminol- and lucigenin-dependent chemiluminescence. J. Cell. Physiol. 1992, 151, 466–477. [Google Scholar]
- Lukoseviciute, K.; Zilinskas, H.; Januskauskas, A. Effect of exogenous progesterone on post-thaw capacitation and acrosome reaction of bovine sperm. Reprod. Domest. Anim. 2004, 39, 154–161. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Donà, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of Astaxanthin on Human Sperm Capacitation. Mar. Drugs 2013, 11, 1909-1919. https://doi.org/10.3390/md11061909
Donà G, Kožuh I, Brunati AM, Andrisani A, Ambrosini G, Bonanni G, Ragazzi E, Armanini D, Clari G, Bordin L. Effect of Astaxanthin on Human Sperm Capacitation. Marine Drugs. 2013; 11(6):1909-1919. https://doi.org/10.3390/md11061909
Chicago/Turabian StyleDonà, Gabriella, Ivana Kožuh, Anna Maria Brunati, Alessandra Andrisani, Guido Ambrosini, Guglielmo Bonanni, Eugenio Ragazzi, Decio Armanini, Giulio Clari, and Luciana Bordin. 2013. "Effect of Astaxanthin on Human Sperm Capacitation" Marine Drugs 11, no. 6: 1909-1919. https://doi.org/10.3390/md11061909
APA StyleDonà, G., Kožuh, I., Brunati, A. M., Andrisani, A., Ambrosini, G., Bonanni, G., Ragazzi, E., Armanini, D., Clari, G., & Bordin, L. (2013). Effect of Astaxanthin on Human Sperm Capacitation. Marine Drugs, 11(6), 1909-1919. https://doi.org/10.3390/md11061909