Alkaloids from the Mangrove-Derived Actinomycete Jishengella endophytica 161111
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structure Elucidation




| Position | 3 | 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | |
| 2 | 167.5, qC | 166.7, qC | 166.5, qC | 167.2, qC | ||||
| 3 | 56.4, CH | 5.65, dd, (5.7, 10.2) | 63.1, CH | 5.36, d, (8.0) | 62.0, CH | 5.46, d, (6.9) | 59.0, CH | 5.84, t, (5.6) |
| 5 | 130.7, qC | 131.4, qC | 131.2, qC | 130.7, qC | ||||
| 6 | 125.2, CH | 7.14, d, (4.0) | 125.4, CH | 7.17, d, (4.0) | 125.6, CH | 7.2, d, (3.8) | 125.4, CH | 7.19, d, (4.0) |
| 7 | 107.2, CH | 6.31, d, (4.0) | 107.2, CH | 6.34, d, (4.0) | 107.1, CH | 6.34, d, (3.8) | 106.4, CH | 6.17, d, (4.0) |
| 8 | 132.5, qC | 132.7, qC | 132.8, qC | 132.4, qC | ||||
| 9 | 63.7, CH2 | 5.70, d, (15.2) | 64.2, CH2 | 5.64, d, (15.5) | 64.4, CH2 | 5.64, d, (15.6) | 63.6, CH2 | 5.24, d, (15.1) |
| 5.51, d, (15.2) | 5.53, d, (15.5) | 5.54, d, (15.6) | 4.06, d, (15.1) | |||||
| 10 | 180.1, CH | 9.49, s | 180.2, CH | 9.51, s | 180.0, CH | 9.5, s | 179.9, CH | 9.51, s |
| 11 | 41.3, CH2 | 2.00, ddd, (4.3, 10.2, 13.4) | 32.4, CH | 2.33, m | 39.3, CH | 2.08, m | 39.5, CH2 | 3.33, m |
| 1.60, ddd, (5.7, 9.3, 13.4) | ||||||||
| 12 | 24.7, CH | 1.66, m | 19.3, CH3 | 0.97, d, (6.8) | 25.8, CH2 | 1.39, m; 1.25, m | 135.1, qC | |
| 13 | 21.6, CH3 | 1.00, d, (6.4) | 18.8, CH3 | 0.92, d, (6.8) | 15.5, CH3 | 0.90, d, (7.0) | 129.8, CH | 6.85, d, (7.3) |
| 14 | 23.3, CH3 | 0.89, d, (6.4) | 11.8, CH3 | 0.91, t, (7.1) | 129.1, CH | 7.25, t, (7.3) | ||
| 15 | 128.2, CH | 7.29, t, (7.3) | ||||||
| 16 | 129.1, CH | 7.25, t, (7.3) | ||||||
| 17 | 129.8, CH | 6.85, d, (7.3) | ||||||
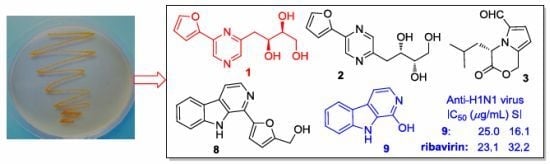
2.2. The Bioactivities of Compounds 1–13 from J. endophytica 161111
3. Experimental Section
3.1. General Experimental Procedures
3.2. Actinomycetes Material
3.3. Fermentation and Extraction
3.4. Purification and Identification
| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δH, Mult. (J in Hz, −4 °C) | δC, Type | δH, Mult. (J in Hz) | δH, Mult. (J in Hz, −4 °C) | |
| 2 | 144.2, qC | 142.5, qC | ||||
| 3 | 136.7, CH | 8.78, s | 8.79, s | 138.7, CH | 8.90, d, (0.7) | 8.91, d, (1.0) |
| 5 | 142.7, CH | 8.38, s | 8.38, s | 153.4, qC | ||
| 6 | 155.3, qC | 144.3, CH | 8.50, d, (0.7) | 8.50, d, (1.0) | ||
| 2′ | 151.1, qC | 151.0, qC | ||||
| 3′ | 110.5, CH | 7.23, d, (4.0) | 7.25, d, 3.4 | 109.9, CH | 7.18, d, (3.4) | 7.19,d, (3.4) |
| 4′ | 112.0, CH | 6.63, dd, (2.1, 4.0) | 6.64, dd, (1.7, 3.4) | 111.9, CH | 6.63, d, (1.7, 3.4) | 6.64, dd, (1.7, 3.4) |
| 5′ | 144.6, CH | 7.72, d, (2.1) | 7.74, d, (1.7) | 144.6, CH | 7.71, d, (1.7) | 7.73, d, (1.7) |
| 1″ | 38.4, CH2 | 3.24, dd, (3.1, 14.0) | 3.24, dd, (2.9, 14.0) | 38.3, CH2 | 3.22, dd, (2.8, 14.0) | 3.22, dd, (2.8, 14.0) |
| 2.93, dd, (9.4, 14.0) | 2.91, dd, (9.7, 14.0) | 2.93, dd, (9.4, 14.0) | 2.91, dd, (9.5, 14.1) | |||
| 2″ | 71.5, CH | 4.03, ddd, (3.1, 7.0, 9.4) | 4.01, ddd, (2.9, 7.2, 9.7) | 71.6, CH | 3.97, ddd, (2.8, 7.0, 9.4) | 3.95, ddd, (2.8, 7.1, 9.5) |
| 3″ | 74.8, CH | 3.58, ddd, (3.8, 7.0, 6.2) | 3.57, ddd, (3.8, 7.2, 6.5) | 74.8, CH | 3.57, (3.7, 7.0, 6.5) | 3.56, ddd, (3.7, 7.1, 6.5) |
| 4″ | 63.2, CH2 | 3.80, dd, (3.8, 11.4) | 3.80, dd, (3.8, 11.2) | 63.2, CH2 | 3.79, dd, (3.7, 11.3) | 3.78, dd, (3.7, 11.3) |
| 3.65, dd, (6.2, 11.4) | 3.63, dd, (6.5, 11.2) | 3.64, dd, (6.2, 11.3) | 3.62, dd, (6.5, 11.3) | |||
3.5. Bioassays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove Plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Hong, K.; Gao, A.H.; Xie, Q.Y.; Gao, H.; Zhuang, L.; Lin, H.P.; Yu, H.P.; Li, J.; Yao, X.S.; Goodfellow, M.; et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef]
- Fu, P.; Yang, C.L.; Wang, Y.; Liu, P.P.; Ma, Y.M.; Xu, L.; Su, M.B.; Hong, K.; Zhu, W.M. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomyces sp. F MA. Org. Lett. 2012, 14, 2422–2425. [Google Scholar] [CrossRef]
- Ara, I.; Kudo, T.; Matsumoto, A.; Takahashi, Y.; Omura, S. Nonomuraea maheshkhaliensis sp. nov., a novel actinomycete isolated from mangrove rhizosphere mud. J. Gen. Appl. Microbiol. 2007, 53, 159–166. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Wang, C.; Wang, R.; Qu, Z.; Lin, H.P.; Goodfellow, M.; Hong, K. Jishengella endophytica gen. nov., sp. nov., a new member of the family Micromonosporaceae. Int. J. Syst. Evol. Microbiol. 2011, 61, 1153–1159. [Google Scholar] [CrossRef]
- Wu, X.A.; Zhao, Y.M.; Yu, N.J. A novel analgesic pyrazine derivative from the leaves of Crotontiglium L. J. Asian Nat. Prod. Res. 2007, 9, 451–455. [Google Scholar]
- Ding, Z.G.; Zhao, J.Y.; Yang, P.W.; Li, M.G.; Huang, R.; Cui, X.L.; Wen, M.L. 1H and 13C NMR assignments of eight nitrogen containing compounds from Nocardia alba sp.nov (YIM 30243T). Magn. Reson. Chem. 2009, 47, 366–370. [Google Scholar] [CrossRef]
- Sannai, Y.; Fujimori, T.; Kato, K. Studies on flavor components of roasted chicory root. Agric. Biol. Chem. 1982, 46, 429–433. [Google Scholar] [CrossRef]
- Tressl, R.; Nittka, C.; Kersten, E. Formation of isoleucine-specific maillard products from [l-13C]-d-glucose and [l-13C]-d-fructose. J. Agric. Food Chem. 1995, 43, 1163–1169. [Google Scholar] [CrossRef]
- Jeric, I.; Simicic, L.; Stipetic, M.; Horvat, S. Synthesis and reactivity of the monosaccharide esters of amino acids as models of teichoic acid fragment. Glycoconj. J. 2000, 17, 273–282. [Google Scholar] [CrossRef]
- Su, B.N.; Chang, L.C.; Park, E.J.; Cuendet, M.; Santarsiero, B.D.; Mesecar, A.D.; Mehta, R.G.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Bioactive constituents of the seeds of Brucea javanica. Planta Med. 2002, 68, 730–733. [Google Scholar] [CrossRef]
- Dassonneville, B.; Witulski, B.; Detert, H. [2+2+2] Cycloadditions of alkynylynamides—A total synthesis of perlolyrine and the first total synthesis of “isoperlolyrine”. Eur. J. Org. Chem. 2011, 2011, 2836–2844. [Google Scholar] [CrossRef]
- Jiao, W.H.; Gao, H.; Li, C.Y.; Zhou, G.X.; Kitanaka, S.; Ohmura, A.; Yao, X.S. β-Carboline alkaloids from the stems of Picrasma quassioides. Magn. Reson. Chem. 2010, 48, 490–495. [Google Scholar]
- Ashour, M.A.; Elkhayat, E.S.; Ebel, R.; Edrada, R.; Proksch, P. Indole alkaloid from the red sea sponge Hyrtios erectus. Arkivoc 2007, 2007, 225–231. [Google Scholar] [CrossRef]
- Yang, S.W.; Cordell, G.A. Metabolism studies of indole derivatives using a staurosporine producer, Streptomyces staurosporeus. J. Nat. Prod. 1997, 60, 44–48. [Google Scholar] [CrossRef]
- Don, M.J.; Shen, C.C.; Lin, Y.L.; Syu, W.J.; Ding, Y.H.; Sun, C.M. Nitrogen-containing compounds from Salvia miltiorrhiza. J. Nat. Prod. 2005, 68, 1066–1070. [Google Scholar] [CrossRef]
- Duran, R.; Zubia, E.; Ortega, M.J.; Naranjo, S.; Salva, J. Novel alkaloids from the red ascidian Botryllus leachi. Tetrahedron 1999, 55, 13225–13232. [Google Scholar] [CrossRef]
- Takahashi, Y.; Iinuma, Y.; Kubota, T.; Tsuda, M.; Sekiguchi, M.; Mikami, Y.; Fromont, J.; Kobayash, J. Hyrtioseragamines A and B, new alkaloids from the sponge Hyrtios species. Org. Lett. 2011, 13, 628–631. [Google Scholar] [CrossRef]
- Pettit, G.R.; Inoue, M.; Kamano, Y.; Herald, D.L.; Arm, C.; Dufresne, C.; Christie, N.D.; Schmidt, J.M.; Doubek, D.L.; Krupa, T.S. Isolation and structure of the powerful cell growth inhibitor cephalostatin 1. J. Am. Chem. Soc. 1988, 110, 2006–2007. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Xu, J.P.; Ichihara, Y.; Williams, M.D.; Boyd, M.R. Antineoplastic agents. 398. Isolation and structure elucidation of cephalostatins 18 and 19. J. Nat. Prod. 1998, 61, 955–958. [Google Scholar] [CrossRef]
- Pettit, G.R.; Xu, J.P.; Williams, M.D.; Christie, N.D.; Doubek, D.L.; Schmidt, J.M. Isolation and structure of cephalostatins 10 and 11. J. Nat. Prod. 1994, 57, 52–63. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Zou, K. Xylogranatinin, a new pyrido[1, 2-a] pyrazine alkaloid from the fruit of a chinese mangrove Xylocarpus granatum. Chem. Nat. Compd. 2007, 43, 426–428. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, G.Y.; Chen, X.; Huang, M.Z.; Wan, X.Q. Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 2011, 47, 767–769. [Google Scholar] [CrossRef]
- Yang, J.X.; Huang, R.M.; Qiu, S.X.; She, Z.G.; Lin, Y.C. A new isobenzofuranone from the mangrove endophytic fungus Penicillium sp. (ZH58). Nat. Prod. Res. 2013, 27, 1902–1905. [Google Scholar] [CrossRef]
- Ishida, J.; Wang, H.K.; Bastow, K.F.; Hu, C.Q.; Lee, K.H. Antitumor agents 201. 1 Cytotoxicity of harmine and β-carboline analogs. Bioorg. Med. Chem. Lett. 1999, 9, 3319–3324. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tang, J.G.; Wang, R.R.; Yang, L.M.; Dong, Z.J.; Du, L.; Shen, X.; Liu, J.K.; Zheng, Y.T. Flazinamide, a novelb-carboline compound with anti-HIV actions. Biochem. Biophys. Res. Commun. 2007, 355, 1091–1095. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Kobayashi, J.; Harbour, G.C.; Hughes, R.G., Jr.; Mizsak, S.A.; Scahil, T.A. Eudistomins C, E, K, and L, potent antiviral compounds containing a novel oxathiazepine ring from the caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984, 106, 1524–1526. [Google Scholar] [CrossRef]
- Hee Jae, S.; Lee, H.S.; Lee, D.S. The synergistic antibacterial activity of 1-acetyl-β-carboline and β-lactams against methicillin-resistant Staphylococcus aureus(MRSA). J. Microbiol. Biotechnol. 2010, 20, 501–505. [Google Scholar]
- Rao, K.V.; Santarsiero, B.D.; Mesecar, A.D.; Schinazi, R.F.; Tekwani, B.L.; Hamann, M.T. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an indonesian sponge. J. Nat. Prod. 2003, 66, 823–828. [Google Scholar] [CrossRef]
- Li, L.; Cui, G.H.; Zhao, M.; Wang, Y.J.; Wang, H.; Li, W.; Peng, S.Q. Assembly of β-cyclodextrin with 3S-tetrahydro-β-carboline-3-carboxylic acid and self-assembly of 6-(3′S-carboline-3′-carboxylamino ethylamino)-6-deoxy-β-cyclodextrin: Approaches to enhance anti-oxidation stability and anti-thrombotic potency. J. Phys. Chem. B 2008, 112, 12139–12147. [Google Scholar] [CrossRef]
- Ding, L.; Dahse, H.M.; Hertweck, C. Cytotoxic alkaloids from Fusarium incarnatum associated with the mangrove tree Aegiceras corniculatum. J. Nat. Prod. 2012, 75, 617–621. [Google Scholar]
- Sandler, J.S.; Colin, P.L.; Hooper, J.N.A.; John Faulkner, D. Cytotoxic β-carbolines and cyclic Peroxides from the palauan sponge Plakortis nigra. J. Nat. Prod. 2002, 65, 1258–1261. [Google Scholar] [CrossRef]
- Schupp, P.; Poehner, T.; Edrada, R.; Ebel, R.; Berg, A.; Wray, V.; Proksch, P. Eudistomins W and X, two new β-carbolines from the micronesian tunicate Eudistoma sp. J. Nat. Prod. 2003, 66, 272–275. [Google Scholar] [CrossRef]
- Wang, W.H.; Nam, S.J.; Lee, B.C.; Kang, H. β-carboline alkaloids from a korean tunicate Eudistoma sp. J. Nat. Prod. 2008, 71, 163–166. [Google Scholar] [CrossRef]
- Marisa, T.; Michele, R.P. 5-bromo-8-methoxy-1-methyl-β-carboline, an alkaloid from the New Zealand marine bryozoan Pterocella Wesiculosa. J. Nat. Prod. 2009, 72, 796–798. [Google Scholar] [CrossRef]
- Harwood, D.T.; Urban, S.; Blunt, J.W.; Munro, M.H.G. β-carboline alkaloids from a new zealand marine bryozoan, Cribricellina Cribraria. Nat. Prod. Res. 2003, 17, 15–19. [Google Scholar] [CrossRef]
- Cabrera, G.M.; Seldes, A.M. A β-carboline alkaloid from the soft coral Lignopsis spongiosum. J. Nat. Prod. 1999, 62, 759–760. [Google Scholar] [CrossRef]
- Penez, N.; Culioli, G.; Perez, T.; Briand, J.F.; Thomas, O.P.; Blache, Y. Antifouling properties of simple indole and purine alkaloids from the mediterranean gorgonian Paramuricea clavata. J. Nat. Prod. 2011, 74, 2304–2308. [Google Scholar] [CrossRef]
- Huang, H.B.; Yao, Y.L.; He, Z.X.; Yang, T.T.; Ma, J.Y.; Tian, X.P.; Li, Y.Y.; Huang, C.G.; Chen, X.P.; Li, W.J.; et al. Antimalarial β-carboline and indolactam alkaloids from Marinactinospora thermotolerans, a deep sea isolate. J. Nat. Prod. 2011, 7, 2122–2127. [Google Scholar]
- Zheng, L.; Chen, H.M.; Han, X.T.; Lin, W.; Yan, X.J. Antimicrobial screening and active compound isolation from marine bacterium NJ6–3-1 associated with the sponge Hymeniacidon perleve. World J. Microbol. Biotechnol. 2005, 21, 201–206. [Google Scholar] [CrossRef]
- Seeman, J.I.; Paine, J.B., III; Secor, H.V.; Im, H.S.; Bernstein, E.R. Supersonic jet studies of alkyl substituted pyrazines and pyridines. Minimum-energy conformations and torsional motion. J. Am. Chem. Soc. 1992, 114, 5269–5280. [Google Scholar] [CrossRef]
- Matsumori, N.; Kaneno, D.; Murata, M.; Nakamura, H.; Tachibana, K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 1999, 64, 866–876. [Google Scholar] [CrossRef]
- Stephens, P.J.; Pan, J.J. Determination of the absolute configurations of pharmacological natural products via density functional theory calculations of vibrational circular dichroism: The new cytotoxic iridoid prismatomerin. J. Org. Chem. 2007, 72, 7641–7649. [Google Scholar] [CrossRef]
- Jexkings, J.P.; Klyne, W.; Scope, P.M. Optical rotatory dispersion. Part XXIV. Lactones. J. Chem. Soc. 1965, 7211–7229. [Google Scholar] [CrossRef]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef]
- Hung, H.C.; Tseng, C.P.; Yang, J.M.; Ju, Y.W.; Tseng, S.N.; Chen, Y.F.; Chao, Y.S.; Hsieh, H.P.; Shih, S.R.; Hsu, J.T. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antivir. Res. 2009, 81, 123–131. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tang, J.G.; Wang, Y.H.; Wang, R.R.; Dong, Z.J.; Yang, L.M.; Zheng, Y.T.; Liu, J.K. Synthesis of analogues of flazin, in particular, flazinamide, as promising anti-HIV agents. Chem. Biodivers. 2008, 5, 447–460. [Google Scholar] [CrossRef]
- Nazari Formagio, A.S.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Dusman Tonin, L.T.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and antiviral activity of β-carboline derivatives bearing a substituted carbohydrazide at C-3 against poliovirus and herpes simplex virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef]
- Van Maarseveen, J.H.; Scheeren, H.W.; Clercq, E.D.; Balzarini, J.; Kruse, C.G. Antiviral and Ttumor cell antiproliferative SAR studies on tetracyclic eudistomins II. Bioorg. Med. Chem. 1997, 5, 955–970. [Google Scholar] [CrossRef]
- Van Maameveen, J.H.; Hermkens, P.H.H.; Clercq, E.D.; Balzarini, J.; Scheeren, H.W.; Kruse, C.G. Antiviral and antitumor structure-activity relationship studies on tetracyclic eudistomines. J. Med. Chem. 1992, 35, 3223–3230. [Google Scholar] [CrossRef]
Supplementary Files
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, P.; Kong, F.; Wei, J.; Wang, Y.; Wang, W.; Hong, K.; Zhu, W. Alkaloids from the Mangrove-Derived Actinomycete Jishengella endophytica 161111. Mar. Drugs 2014, 12, 477-490. https://doi.org/10.3390/md12010477
Wang P, Kong F, Wei J, Wang Y, Wang W, Hong K, Zhu W. Alkaloids from the Mangrove-Derived Actinomycete Jishengella endophytica 161111. Marine Drugs. 2014; 12(1):477-490. https://doi.org/10.3390/md12010477
Chicago/Turabian StyleWang, Pei, Fandong Kong, Jingjing Wei, Yi Wang, Wei Wang, Kui Hong, and Weiming Zhu. 2014. "Alkaloids from the Mangrove-Derived Actinomycete Jishengella endophytica 161111" Marine Drugs 12, no. 1: 477-490. https://doi.org/10.3390/md12010477
APA StyleWang, P., Kong, F., Wei, J., Wang, Y., Wang, W., Hong, K., & Zhu, W. (2014). Alkaloids from the Mangrove-Derived Actinomycete Jishengella endophytica 161111. Marine Drugs, 12(1), 477-490. https://doi.org/10.3390/md12010477