Pelagia noctiluca (Scyphozoa) Crude Venom Injection Elicits Oxidative Stress and Inflammatory Response in Rats
Abstract
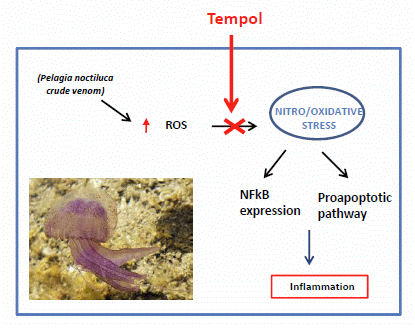
:1. Introduction
2. Results
2.1. Effect of Pelagia noctiluca Crude Venom on Blood Pressure and Mortality

2.2. Effect of Crude Venom on Hepatocellular, Pancreatic and Renal Dysfunction

2.3. Histological Evaluation after Pelagia noctiluca Crude Venom Treatment

2.4. Effect of Pelagia noctiluca Crude Venom on the Degradation of IκB-α and Translocation of NF-kB p65

2.5. Effect of Pelagia noctiluca Crude Venom on COX2 Expression
2.6. Effect of Pelagia noctiluca Crude Venom on P-Selectin and ICAM-1 Expression and MPO Activity

2.7. Effects of Pelagia noctiluca Crude Venom on NO Production
2.8. Effect of Pelagia noctiluca Crude Venom on Nitrotyrosine Production, PARP Activation and malondialdehyde (MDA) Levels
2.9. Effects of Pelagia noctiluca Crude Venom on Apoptotic Proteins Expression



3. Discussion
4. Experimental Section
4.1. Animals
4.2. Nematocysts Isolation and Crude Venom Extraction
4.3. Experimental Groups
- Vehicle: group treated with saline alone. (n = 25 rats/group)
- Crude venom 6 μg/kg + Vehicle (saline): group intravenously injected with crude venom at the dose of 6 μg/kg. (n = 25 rats/group)
- Crude venom 30 μg/kg + Vehicle (saline): group intravenously injected with crude venom at the dose of 30 μg/kg. (n = 25 rats/group)
- Crude venom 60 μg/kg + Vehicle (saline): group intravenously injected with crude venom at the dose of 60 μg/kg. (n = 25 rats/group)
- Vehicle: group treated only with saline. (n = 25 rats/group)
- Tempol: group treated with tempol alone (100 mg/kg i.v. dissolved in saline). (n = 25 rats/group)
- Crude venom 30 μg/kg + Vehicle (saline): group injected by crude venom at the dose of 30 μg/kg. (n = 25 rats/group)
- Crude venom 30 μg/kg + Tempol: group injected by crude venom at the dose of 30 μg/kg and treated with tempol (100 mg/kg i.v. dissolved in saline) 30 min and 1 h after crude venom injection. (n = 25 rats/group)
4.4. Blood Pressure Measurement
4.5. Myeloperoxidase Activity
4.6. Malondialdehyde (MDA) Measurement
4.7. Measurement of Nitrite/Nitrate
4.8. Histological Examination
4.9. Immunohistochemical Localization of iNOS, Nitrotyrosine, ICAM-1, P-Selectin and Poly ADP-Ribose (PAR)
4.10. Western Blot Analysis for IκB-α, NF-κB p65, iNOS, COX2, Bax, and Bcl-2
4.11. Quantification of Organ Function and Injury
4.12. Materials
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suarez-Jimenez, G.M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. Bioactive peptides and depsipeptides with anticancer potential: Sources from marine animals. Mar. Drugs 2012, 10, 963–986. [Google Scholar]
- Lazcano-Perez, F.; Roman-Gonzalez, S.A.; Sanchez-Puig, N.; Arreguin-Espinosa, R. Bioactive peptides from marine organisms: A short overview. Protein Pept. Lett. 2012, 19, 700–707. [Google Scholar] [CrossRef]
- Castaneda, O.; Harvey, A.L. Discovery and characterization of cnidarian peptide toxins that affect neuronal potassium ion channels. Toxicon 2009, 54, 1119–1124. [Google Scholar] [CrossRef]
- Grotendorst, G.R.; Hessinger, D.A. Enzymatic characterization of the major phospholipase A2 component of sea anemone (Aiptasia pallida) nematocyst venom. Toxicon 2000, 38, 931–943. [Google Scholar] [CrossRef]
- Carli, A.; Bussotti, S.; Mariottini, G.L.; Robbiano, L. Toxicity of jellyfish and sea-anemone venoms on cultured V79 cells. Toxicon 1996, 34, 496–500. [Google Scholar] [CrossRef]
- Marino, A.; Morabito, R.; Pizzata, T.; La Spada, G. Effect of various factors on Pelagia noctiluca (Cnidaria, Scyphozoa) crude venom-induced haemolysis. Comp. Biochem. Physiol. 2008, 151, 144–149. [Google Scholar] [CrossRef]
- Frazao, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef]
- Anderson, P.A.; Bouchard, C. The regulation of cnidocyte discharge. Toxicon 2009, 54, 1046–1053. [Google Scholar] [CrossRef]
- Turk, T.; Kem, W.R. The phylum Cnidaria and investigations of its toxins and venoms until 1990. Toxicon 2009, 54, 1031–1037. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Mediterranean jellyfish venoms: A review on scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; de Girolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef]
- Hodgson, E. Toxins and venoms. Prog. Mol. Biol. Transl. Sci. 2012, 112, 373–415. [Google Scholar] [CrossRef]
- Jain, D.; Kumar, S. Snake venom: A potent anticancer agent. Asian Pac. J. Cancer Prev. 2012, 13, 4855–4860. [Google Scholar] [CrossRef]
- Fenner, P.J.; Harrison, S.L. Irukandji and Chironex fleckeri jellyfish envenomation in tropical Australia. Wilderness Environ. Med. 2000, 11, 233–240. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; da Silveira, F.L.; Cardoso, J.L.; Morandini, A.C. A report of 49 cases of cnidarian envenoming from southeastern Brazilian coastal waters. Toxicon 2002, 40, 1445–1450. [Google Scholar] [CrossRef]
- Marques, A.C.; Haddad, V., Jr.; Esteves Migotto, A. Envenomation by a benthic Hydrozoa (Cnidaria): the case of Nemalecium lighti (Haleciidae). Toxicon 2002, 40, 213–215. [Google Scholar] [CrossRef]
- Marino, A.; Crupi, R.; Rizzo, G.; Morabito, R.; Musci, G.; la Spada, G. The unusual toxicity and stability properties of crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Mol. Biol. 2007, 53, OL994–OL1002. [Google Scholar]
- Marino, A.; di Paola, R.; Crisafulli, C.; Mazzon, E.; Morabito, R.; Paterniti, I.; Galuppo, M.; Genovese, T.; la Spada, G.; Cuzzocrea, S. Protective effect of melatonin against the inflammatory response elicited by crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). J. Pineal Res. 2009, 47, 56–69. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Zheng, J.; Wang, Q.; Wang, T.; Lu, J.; Wen, X.; Zhang, B.; Liu, G.; Zhang, W.; et al. Multiple organ dysfunction: A delayed envenomation syndrome caused by tentacle extract from the jellyfish Cyanea capillata. Toxicon 2013, 61, 54–61. [Google Scholar] [CrossRef]
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of cytotoxicity of Pelagia noctiluca venom causes reactive oxygen species generation, lipid peroxydation induction and DNA damage in human colon cancer cells. Lipids Health Dis. 2011, 10, 232. [Google Scholar] [CrossRef]
- Sher, D.; Fishman, Y.; Zhang, M.; Lebendiker, M.; Gaathon, A.; Mancheno, J.M.; Zlotkin, E. Hydralysins, a new category of beta-pore-forming toxins in cnidaria. J. Biol. Chem. 2005, 280, 22847–22855. [Google Scholar]
- Monroy-Estrada, H.I.; Segura-Puertas, L.; Galvan-Arzate, S.; Santamaria, A.; Sanchez-Rodriguez, J. The crude venom from the sea anemone Stichodactyla helianthus induces haemolysis and slight peroxidative damage in rat and human erythrocytes. Toxicol. In Vitro 2007, 21, 398–402. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar]
- Ischiropoulos, H.; Zhu, L.; Beckman, J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992, 298, 446–451. [Google Scholar] [CrossRef]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995, 268, (5 Pt 1). L699–L722. [Google Scholar]
- Szabo, C.; Zingarelli, B.; Salzman, A.L. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ. Res. 1996, 78, 1051–1063. [Google Scholar] [CrossRef]
- Szabo, C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock 1996, 6, 79–88. [Google Scholar] [CrossRef]
- Szabo, C.; Salzman, A.L.; Ischiropoulos, H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995, 372, 229–232. [Google Scholar] [CrossRef]
- Szabo, C.; Salzman, A.L.; Ischiropoulos, H. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 1995, 363, 235–238. [Google Scholar] [CrossRef]
- Zingarelli, B.; Day, B.J.; Crapo, J.D.; Salzman, A.L.; Szabo, C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol. 1997, 120, 259–267. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Krishna, M.C.; Russo, A.; Mitchell, J.B.; Goldstein, S.; Dafni, H.; Samuni, A. Do nitroxide antioxidants act as scavengers of O2−. or as SOD mimics? J. Biol. Chem. 1996, 271, 26026–26031. [Google Scholar]
- Mitchell, J.B.; Samuni, A.; Krishna, M.C.; DeGraff, W.G.; Ahn, M.S.; Samuni, U.; Russo, A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry 1990, 29, 2802–2807. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Simonsen, U.; Christensen, F.H.; Buus, N.H. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol. Ther. 2009, 122, 109–124. [Google Scholar] [CrossRef]
- Sasaki, H.; Lin, L.R.; Yokoyama, T.; Sevilla, M.D.; Reddy, V.N.; Giblin, F.J. TEMPOL protects against lens DNA strand breaks and cataract in the X-rayed rabbit. Investig. Ophthalmol. Vis. Sci. 1998, 39, 544–552. [Google Scholar]
- Kishimoto, N.; Yamamoto, I.; Toraishi, K.; Yoshioka, S.; Saito, K.; Masuda, H.; Fujita, T. Two distinct pathways for the formation of hydroxy FA from linoleic acid by lactic acid bacteria. Lipids 2003, 38, 1269–1274. [Google Scholar] [CrossRef]
- Metz, J.M.; Smith, D.; Mick, R.; Lustig, R.; Mitchell, J.; Cherakuri, M.; Glatstein, E.; Hahn, S.M. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004, 10, 6411–6417. [Google Scholar] [CrossRef]
- Noguchi, K.; Sakanashi, M.; Matsuzaki, T.; Nakasone, J.; Sakanashi, M.; Koyama, T.; Hamadate, N.; Sakanashi, M. Cardiovascular effects and lethality of venom from nematocysts of the box-jellyfish Chiropsalmus quadrigatus (Habu-kurage) in anaesthetized rats. Toxicon 2005, 45, 519–526. [Google Scholar] [CrossRef]
- Burnett, J.W. Treatment of Atlantic cnidarian envenomations. Toxicon 2009, 54, 1201–1205. [Google Scholar] [CrossRef]
- Anderluh, G.; Macek, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef]
- Shiomi, K. Novel peptide toxins recently isolated from sea anemones. Toxicon 2009, 54, 1112–1118. [Google Scholar] [CrossRef]
- Suput, D. In vivo effects of cnidarian toxins and venoms. Toxicon 2009, 54, 1190–1200. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Sottofattori, E.; Mazzei, M.; Robbiano, L.; Carli, A. Cytotoxicity of the venom of Pelagia noctiluca forskal (Cnidaria: Scyphozoa). Toxicon 2002, 40, 695–698. [Google Scholar] [CrossRef]
- Morabito, R.; Condello, S.; Curro, M.; Marino, A.; Ientile, R.; la Spada, G. Oxidative stress induced by crude venom from the jellyfish Pelagia noctiluca in neuronal-like differentiated SH-SY5Y cells. Toxicol. In Vitro 2012, 26, 694–699. [Google Scholar] [CrossRef]
- Yim, M.B.; Chock, P.B.; Stadtman, E.R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc. Natl. Acad. Sci. USA 1990, 87, 5006–5010. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Haddad, J.J. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell. Signal. 2002, 14, 879–897. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar]
- Byun, M.S.; Jeon, K.I.; Choi, J.W.; Shim, J.Y.; Jue, D.M. Dual effect of oxidative stress on NF-κB activation in HeLa cells. Exp. Mol. Med. 2002, 34, 332–339. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. 2007, 7, 678–689. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Le Bras, M.; Clement, M.V.; Pervaiz, S.; Brenner, C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol. Histopathol. 2005, 20, 205–219. [Google Scholar]
- Ayed, Y.; Bouaziz, C.; Brahmi, D.; Zaid, C.; Abid, S.; Bacha, H. Cell death in relation to DNA damage after exposure to the jellyfish Pelagia noctiluca nematocysts. Environ. Toxicol. 2014, 29, 337–344. [Google Scholar] [CrossRef]
- Mariscal, R.N. Scanning electron microscopy of the sensory surface of the tentacles of sea anemones and corals. Z Zellforsch Mikrosk Anat 1974, 147, 149–156. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; McDonald, M.C.; Filipe, H.M.; Costantino, G.; Mazzon, E.; Santagati, S.; Caputi, A.P.; Thiemermann, C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur. J. Pharmacol. 2000, 390, 209–222. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Esposito, E.; Muia, C.; Abdelrahman, M.; di Paola, R.; Crisafulli, C.; Bramanti, P.; Thiemermann, C. Glycogen synthase kinase-3beta inhibition attenuates the development of ischaemia/reperfusion injury of the gut. Intensive Care Med. 2007, 33, 880–893. [Google Scholar] [CrossRef]
- Bethea, J.R.; Castro, M.; Keane, R.W.; Lee, T.T.; Dietrich, W.D.; Yezierski, R.P. Traumatic spinal cord injury induces nuclear factor-κB activation. J. Neurosci. 1998, 18, 3251–3260. [Google Scholar]
- Chatterjee, P.K.; Cuzzocrea, S.; Brown, P.A.; Zacharowski, K.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bruschetta, G.; Impellizzeri, D.; Morabito, R.; Marino, A.; Ahmad, A.; Spanò, N.; Spada, G.L.; Cuzzocrea, S.; Esposito, E. Pelagia noctiluca (Scyphozoa) Crude Venom Injection Elicits Oxidative Stress and Inflammatory Response in Rats. Mar. Drugs 2014, 12, 2182-2204. https://doi.org/10.3390/md12042182
Bruschetta G, Impellizzeri D, Morabito R, Marino A, Ahmad A, Spanò N, Spada GL, Cuzzocrea S, Esposito E. Pelagia noctiluca (Scyphozoa) Crude Venom Injection Elicits Oxidative Stress and Inflammatory Response in Rats. Marine Drugs. 2014; 12(4):2182-2204. https://doi.org/10.3390/md12042182
Chicago/Turabian StyleBruschetta, Giuseppe, Daniela Impellizzeri, Rossana Morabito, Angela Marino, Akbar Ahmad, Nunziacarla Spanò, Giuseppa La Spada, Salvatore Cuzzocrea, and Emanuela Esposito. 2014. "Pelagia noctiluca (Scyphozoa) Crude Venom Injection Elicits Oxidative Stress and Inflammatory Response in Rats" Marine Drugs 12, no. 4: 2182-2204. https://doi.org/10.3390/md12042182
APA StyleBruschetta, G., Impellizzeri, D., Morabito, R., Marino, A., Ahmad, A., Spanò, N., Spada, G. L., Cuzzocrea, S., & Esposito, E. (2014). Pelagia noctiluca (Scyphozoa) Crude Venom Injection Elicits Oxidative Stress and Inflammatory Response in Rats. Marine Drugs, 12(4), 2182-2204. https://doi.org/10.3390/md12042182