The Effect of Polyunsaturated Aldehydes on Skeletonema marinoi (Bacillariophyceae): The Involvement of Reactive Oxygen Species and Nitric Oxide
Abstract
:1. Introduction
2. Results
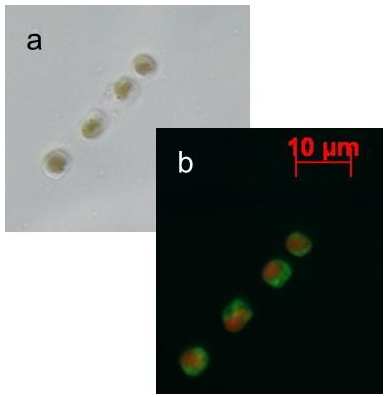
2.1. Nitric Oxide Production

| Concentration (μM) | DECA | OCTA | HEPTA | MIX |
|---|---|---|---|---|
| 0.05 | * | ns | ns | ns |
| 0.1 | * | ns | ns | ns |
| 1 | * | * | ns | ns |
| 5 | * | * | * | ns |
| 10 | - | * | * | * |
| 20 | - | * | * | * |
| 33 | * | - | - | - |
| 40 | - | - | * | - |
| 66 | * | - | - | - |
| cPTIO | * | * | * | * |

2.2. ROS Production


2.3. Xanthophyll Cycle Activation and Photosynthetic Efficiency
| Treatment | Time (h) | Ddx | Dtx | DES | β-carotene | NPQ | μ (day−1) |
|---|---|---|---|---|---|---|---|
| 5 µM OCTA | 0 | 1.07 ± 0.14 | 0.95 ± 0.18 | 0.91 ± 0.23 | 1.07 ± 0.05 | - | - |
| 0.3 | 0.51 ± 0.09 | 0.79 ± 0.10 | 1.52 ± 0.27 | 0.63 ± 0.08 | 0.95 ± 0.04 | - | |
| 1 | 1.29 ± 0.11 | 1.51 ± 0.25 | 1.30 ± 0.31 | 1.00 ± 0.09 | 0.88 ± 0.03 | - | |
| 3 | 2.36 ± 0.51 | 1.57 ± 0.51 | 0.95 ± 0.49 | 1.30 ± 0.48 | 0.88 ± 0.02 | - | |
| 24 | 0.59 ± 0.07 | 1.20 ± 0.36 | 1.86 ± 0.63 | 0.70 ± 0.07 | 1.10 ± 0.04 | 0.42 ± 0.08 | |
| 48 | - | - | - | - | - | −0.19 ± 0.12 | |
| 10 µM OCTA | 0 | 0.84 ± 0.03 | 0.89 ± 0.13 | 1.05 ± 0.17 | 1.00 ± 0.06 | - | - |
| 0.3 | 0.48 ± 0.11 | 0.80 ± 0.15 | 1.61 ± 0.17 | 0.61 ± 0.11 | 0.92 ± 0.03 | - | |
| 1 | 1.37 ± 0.14 | 1.77 ± 0.04 | 1.50 ± 0.30 | 0.95 ± 0.12 | 0.95 ± 0.00 | - | |
| 3 | 1.21 ± 0.11 | 2.14 ± 1.52 | 1.78 ± 0.64 | 1.20 ± 0.30 | 1.00 ± 0.03 | - | |
| 24 | 1.23 ± 0.58 | 1.76 ± 1.17 | 2.37 ± 0.42 | 1.08 ± 0.25 | 1.25 ± 0.04 | 0.11 ± 0.15 | |
| 48 | - | - | - | - | - | −0.12 ± 0.13 | |
| 20 µM OCTA | 0 | 0.75 ± 0.12 | 0.84 ± 0.07 | 1.12 ± 0.20 | 0.89 ± 0.01 | - | - |
| 0.3 | 0.63 ± 0.22 | 1.10 ± 0.01 | 1.90 ± 0.46 | 0.63 ± 0.16 | 0.90 ± 0.17 | - | |
| 1 | 0.82 ± 0.10 | 2.19 ± 0.05 | 1.80 ± 0.37 | 0.87 ± 0.16 | 1.01 ± 0.04 | - | |
| 3 | 2.28 ± 0.46 | 4.51 ± 1.57 | 1.41 ± 0.38 | 1.56 ± 0.46 | 1.05 ± 0.00 | - | |
| 24 | 1.71 ± 0.49 | 5.83 ± 1.60 | 2.88 ± 0.17 | 2.04 ± 0.23 | 1.32 ± 0.02 | −0.29 ± 0.08 | |
| 48 | - | - | - | - | - | −0.10 ± 0.10 |
2.4. Growth and Recovery from PUA Stress
| Treatment | Time (h) | Ddx | Dtx | DES | β-carotene | μ (day−1) |
|---|---|---|---|---|---|---|
| 5 µM OCTA | 24 | 0.80 ± 0.25 | 0.82 ± 0.53 | 0.92 ± 0.38 | 0.88 ± 0.18 | 0.69 ± 0.10 |
| 48 | - | - | - | - | 0.34 ± 0.12 | |
| 10 µM OCTA | 24 | 1.22 ± 0.76 | 1.20 ± 0.76 | 0.98 ± 0.04 | 0.92 ± 0.27 | 0.57 ± 0.04 |
| 48 | - | - | - | - | 0.46 ± 0.08 | |
| 20 µM OCTA | 24 | 2.79 ± 0.26 | 3.30 ± 0.12 | 1.00 ± 0.17 | 2.34 ± 0.28 | 0.09 ± 0.07 |
| 48 | - | - | - | - | 0.47 ± 0.11 |
3. Discussion
3.1. Protective Responses

3.2. Growth
4. Experimental Section
4.1. Culture Conditions and Experimental Design
4.2. PUA
4.3. NO and ROS Detection
4.4. Flow Cytometry
4.5. XC Pigments and Photosynthetic Performance
5. Conclusions
Implications and Ecological Hypotheses
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Wichard, T.; Poulet, S.A.; Halsband-Lenk, C.; Albaina, A.; Harris, R.; Liu, D.Y.; Pohnert, G. Survey of the chemical defence potential of diatoms: Screening of fifty one species for alpha, beta, gamma, delta-unsaturated aldehydes. J. Chem. Ecol. 2005, 31, 949–958. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Caldwell, G.S.; Bentley, M.G.; Olive, P.J.W. The use of a brine shrimp (Artemia salina) bioassay to assess the toxicity of diatom extracts and short chain aldehydes. Toxicon 2003, 42, 301–306. [Google Scholar] [CrossRef]
- Romano, G.; Russo, G.L.; Buttino, I.; Ianora, A.; Miralto, A. A marine diatom-derived aldehyde induces apoptosis in copepod and sea urchin embryos. J. Exp. Biol. 2003, 206, 3487–3494. [Google Scholar] [CrossRef]
- Buttino, I.; Santo, M.D.; Ianora, A.; Miralto, A. Rapid assessment of copepod (Calanus helgolandicus) embryo viability using fluorescent probes. Mar. Biol. 2004, 145, 393–399. [Google Scholar]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Ribalet, F.; Intertaglia, L.; Lebaron, P.; Casotti, R. Differential effect of three polyunsaturated aldehydes on marine bacterial isolates. Aquat. Toxicol. 2008, 86, 249–255. [Google Scholar] [CrossRef]
- Balestra, C.; Alonso-Saez, L.; Gasol, J.M.; Casotti, R. Group-specific effects on coastal bacterioplankton of polyunsaturated aldehydes produced by diatoms. Aquat. Microb. Ecol. 2011, 63, 123–131. [Google Scholar] [Green Version]
- Casotti, R.; Mazza, S.; Brunet, C.; Vantrepotte, V.; Ianora, A.; Miralto, A. Growth inhibition and toxicity of the diatom aldehyde 2-trans, 4-trans-decadienal on Thalassiosira weissflogii (Bacillariophyceae). J. Phycol. 2005, 41, 7–20. [Google Scholar] [CrossRef]
- Ribalet, F.; Berges, J.A.; Ianora, A.; Casotti, R. Growth inhibition of cultured marine. Phytoplankton by toxic algal-derived polyunsaturated aldehydes. Aquat. Toxicol. 2007, 85, 219–227. [Google Scholar] [CrossRef]
- Vardi, A.; Formiggini, F.; Casotti, R.; de Martino, A.; Ribalet, F.; Miralto, A.; Bowler, C. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biol. 2006, 4, 411–419. [Google Scholar] [CrossRef]
- Vardi, A.; Bidie, K.D.; Kwityn, C.; Hirsh, D.J.; Thompson, S.M.; Callow, J.A.; Falkowski, P.; Bowler, C. A diatom gene regulating nitric-oxide signaling and susceptibility to diatom-derived aldehydes. Curr. Biol. 2008, 18, 895–899. [Google Scholar]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar]
- Moroz, L.L.; Kohn, A.B. Parallel evolution of nitric oxide signaling: Diversity of synthesis and memory pathways. Front. Biosci. 2011, 17, 2008–2051. [Google Scholar] [CrossRef]
- Wendehenne, D.; Durner, J.; Klessig, D.F. Nitric oxide: A new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 2004, 7, 449–455. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C.; Mohn, F.H.; Soeder, C.J. Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 1999, 39, 1601–1610. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H.; Rai, L.; Soeder, C.J. Impact of physiological stresses on nitric oxide formation by green alga, Scenedesmus obliquus. J. Microbiol. Biotechnol. 2000, 10, 300–306. [Google Scholar]
- Kim, D.; Kang, Y.S.; Lee, Y.; Yamaguchi, K.; Matsuoka, K.; Lee, K.W.; Choi, K.S.; Oda, T. Detection of nitric oxide (NO) in marine phytoplankters. J. Biosci. Bioeng. 2008, 105, 414–417. [Google Scholar] [CrossRef]
- Perez, S.; Weis, V. Nitric oxide and cnidarian bleaching: An eviction notice mediates breakdown of a symbiosis. J. Exp. Biol. 2006, 209, 2804–2810. [Google Scholar] [CrossRef]
- Bouchard, J.N.; Yamasaki, H. Implication of nitric oxide in the heat-stress-induced cell death of the symbiotic alga Symbiodinium microadriaticum. Mar. Biol. 2009, 156, 2209–2220. [Google Scholar] [CrossRef]
- Chung, C.C.; Hwang, S.P.L.; Chang, J. Nitric oxide as a signaling factor to upregulate the death-specific protein in a marine diatom, Skeletonema costatum, during blockage of electron flow in photosynthesis. Appl. Environ. Microbiol. 2008, 74, 6521–6527. [Google Scholar] [CrossRef]
- Thompson, S.E.M.; Taylor, A.R.; Brownlee, C.; Callow, M.E.; Callow, J.A. The role of nitric oxide in diatom adhesion in relation to substratum properties. J. Phycol. 2008, 44, 967–976. [Google Scholar] [CrossRef]
- Leflaive, J.; Ten-Hage, L. Effects of 2E,4E-Decadienal on motility and aggregation of diatoms and on biofilm formation. Microb. Ecol. 2011, 61, 363–373. [Google Scholar] [CrossRef]
- Estevez, M.S.; Puntarulo, S. Nitric oxide generation upon growth of Antarctic Chlorella sp. cells. Physiol. Plant 2005, 125, 192–201. [Google Scholar] [CrossRef]
- Chen, G.F.; Wang, G.C.; Zhang, B.Y.; Fan, X.L. Morphological and phylogenetic analysis of Skeletonema costatum-like diatoms (Bacillariophyta) from the China Sea. Eur. J. Phycol. 2007, 42, 163–175. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Vardi, A.; Berman-Frank, I.; Rozenberg, T.; Hadas, O.; Kaplan, A.; Levine, A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr. Biol. 1999, 9, 1061–1064. [Google Scholar] [CrossRef]
- Evans, C.; Malin, G.; Mills, G.P.; Wilson, W.H. Viral infection of Emiliania huxleyi (Prymnesiophyceae) leads to elevated production of reactive oxygen species. J. Phycol. 2006, 42, 1040–1047. [Google Scholar] [CrossRef]
- Liu, W.; Au, D.W.T.; Anderson, D.M.; Lam, P.K.S.; Wu, R.S.S. Effects of nutrients, salinity, pH and light : Dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Biol. Ecol. 2007, 346, 76–86. [Google Scholar] [CrossRef]
- Bouchard, J.N.; Purdie, D.A. Effect of elevated temperature, darkness, and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. J. Phycol. 2011, 47, 1316–1325. [Google Scholar] [CrossRef]
- Jamers, A.; Lenjou, M.; Deraedt, P.; Van Bockstaele, D.; Blust, R.; de Coen, W. Flow cytometric analysis of the cadmium-exposed green alga Chlamydomonas reinhardtii (Chlorophyceae). Eur. J. Phycol. 2009, 44, 541–550. [Google Scholar] [CrossRef]
- Rioboo, C.; Gonzalez-Barreiro, O.; Abalde, J.; Cid, A. Flow cytometric analysis of the encystment process induced by paraquat exposure in Haematococcus pluvialis (Chlorophyceae). Eur. J. Phycol. 2011, 46, 89–97. [Google Scholar] [CrossRef]
- Vardi, A.; Eisenstadt, D.; Murik, O.; Berman-Frank, I.; Zohary, T.; Levine, A.; Kaplan, A. Synchronization of cell death in a dinoflagellate population is mediated by an excreted thiol protease. Environ. Microbiol. 2007, 9, 360–369. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Olin, M.; Kankaanpaa, H. Oxidative stress response in the red alga Furcellaria lumbricalis (Huds.) Lamour. due to exposure and uptake of the cyanobacterial toxin nodularin from Nodularia spumigena. Harmful Algae 2010, 10, 49–55. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.Y.; Liu, S.P.; Liu, B.Y.; Gao, Y.N.; Wu, Z.B. Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere 2011, 85, 977–982. [Google Scholar] [CrossRef]
- Ross, C.; Kupper, F.C.; Jacobs, R.S. Involvement of reactive oxygen species and reactive nitrogen species in the wound response of Dasycladus vermicularis. Chem. Biol. 2006, 13, 353–364. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Barros, M.P.; Pinto, E.; Sigaud-Kutner, T.C.S.; Cardozo, K.H.M.; Colepicolo, P. Rhythmicity and oxidative/nitrosative stress in algae. Biol. Rhythm. Res. 2005, 36, 67–82. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitao, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Sarno, D.; Balzano, S.; Gu, H.; Andersen, R.A.; Zingone, A. Global diversity and biogeography of Skeletonema species (Bacillariophyta). Protist 2008, 159, 177–193. [Google Scholar] [CrossRef]
- Caldwell, G.S.; Olive, P.J.W.; Bentley, M.G. Inhibition of embryonic development and fertilization in broadcast spawning marine invertebrates by water soluble diatom extracts and the diatom toxin 2-trans,4-trans decadienal. Aquat. Toxicol. 2002, 60, 123–137. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Blackwell Science, Ltd.: Malden, MA, USA, 1997; p. 374. [Google Scholar]
- Zhang, Z.B.; Liu, C.Y.; Wu, Z.Z.; Xing, L.; Li, P.F. Detection of nitric oxide in culture media and studies on nitric oxide formation by marine microalgae. Med. Sci. Monit. 2006, 12, BR75–BR85. [Google Scholar]
- Sarno, D.; Minucci, C.; Stazione Zoologica A. Dohrn, Naples, Italy. Personal communication. 2011.
- Gallina, A.A.; Casotti, R.; Stazione Zoologica A. Dohrn, Naples, Italy. 2012; Unpublished work.
- Vidoudez, C.; Nejstgaard, J.C.; Jakobsen, H.H.; Pohnert, G. Dynamics of dissolved and particulate polyunsaturated aldehydes in mesocosms inoculated with different densities of the diatom Skeletonema marinoi. Mar. Drugs 2011, 9, 345–358. [Google Scholar] [CrossRef]
- Hansen, E.; Eilertsen, H.C. Do the polyunsaturated aldehydes produced by Phaeocystis pouchetii (Hariot) Lagerheim influence diatom growth during the spring bloom in Northern Norway? J. Plankton Res. 2007, 29, 87–96. [Google Scholar] [CrossRef]
- Hansen, E.; Ernstsen, A.; Eilertsen, H.C. Isolation and characterisation of a cytotoxic polyunsaturated aldehyde from the marine phytoplankter Phaeocystis pouchetii (Hariot) Lagerheim. Toxicology 2004, 199, 207–217. [Google Scholar] [CrossRef]
- Chung, C.C.; Hwang, S.P.L.; Chang, J. Cooccurrence of ScDSP gene expression, cell death, and DNA fragmentation in a marine diatom, Skeletonema costatum. Appl. Environ. Microbiol. 2005, 71, 8744–8751. [Google Scholar] [CrossRef]
- Gallina, A.A.; Chung, C.C.; Casotti, R. Expression of Death-Specific Protein genes (DSP) and ROS Production in Response to Polyunsaturated Aldehydes (PUA) in Skeletonema tropicum. Stazione Zoologica A. Dohrn, Naples, Italy and National Taiwan Ocean University: Keelung, Taiwan, Manuscript in preparation; 2014. [Google Scholar]
- Vidoudez, C.; Casotti, R.; Bastianini, M.; Pohnert, G. Quantification of dissolved and particulate polyunsaturated aldehydes in the Adriatic Sea. Mar. Drugs 2011, 9, 500–513. [Google Scholar] [CrossRef] [Green Version]
- Ribalet, F.; Bastianini, M.; Vidoudez, C.; Acri, F.; Berges, J.; Ianora, A.; Miralto, A.; Pohnert, G.; Romano, G.; Wichard, T.; et al. Phytoplankton cell lysis associated with polyunsaturated aldehyde release in the Northern Adriatic Sea. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Brunet, C.; Lavaud, J. Can the xanthophyll cycle help extract the essence of the microalgal functional response to a variable light environment? J. Plankton Res. 2010, 32, 1609–1617. [Google Scholar] [CrossRef]
- Van de Poll, W.H.; van Leeuwe, M.A.; Roggeveld, J.; Buma, A.G.J. Nutrient limitation and high irradiance acclimation reduce PAR and UV-induced viability loss in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). J. Phycol. 2005, 41, 840–850. [Google Scholar] [CrossRef]
- Geider, R.J.; Laroche, J.; Greene, R.M.; Olaizola, M. Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 1993, 29, 755–766. [Google Scholar]
- Bertrand, M.; Schoefs, B.; Siffel, P.; Rohacek, K.; Molnar, I. Cadmium inhibits epoxidation of diatoxanthin to diadinoxanthin in the xanthophyll cycle of the marine diatom Phaeodactylum tricornutum. FEBS Lett. 2001, 508, 153–156. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Evans, C.; Airs, R.L.; Cook, I.; Bale, N.; Wilson, W.H. The response of carotenoids and chlorophylls during virus infection of Emiliania huxleyi (Prymnesiophyceae). J. Exp. Mar. Biol. Ecol. 2007, 344, 101–112. [Google Scholar] [CrossRef]
- Dimier, C.; Corato, F.; Tramontano, F.; Brunet, C. Photoprotection and xanthophyll-cycle activity in three marine diatoms. J. Phycol. 2007, 43, 937–947. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc. Natl. Acad. Sci. USA 1999, 96, 8784–8789. [Google Scholar] [CrossRef]
- Okamoto, O.K.; Pinto, E.; Latorre, L.R.; Bechara, E.J.H.; Colepicolo, P. Antioxidant modulation in response to metal-induced oxidative stress in algal chloroplasts. Arch. Environ. Contam. Toxicol. 2001, 40, 18–24. [Google Scholar] [CrossRef]
- Sigaud-Kutner, T.C.S.; Pinto, E.; Okamoto, O.K.; Latorre, L.R.; Colepicolo, P. Changes in superoxide dismutase activity and photosynthetic pigment content during growth of marine phytoplankters in batch-cultures. Physiol. Plant 2002, 114, 566–571. [Google Scholar] [CrossRef]
- Riseman, S.F.; DiTullio, G.R. Particulate dimethylsulfoniopropionate and dimethylsulfoxide in relation to iron availability and algal community structure in the Peru Upwelling System. Can. J. Fish. Aquat. Sci. 2004, 61, 721–735. [Google Scholar] [CrossRef]
- Khattab, M.M. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: A key role for superoxide anion. Eur. J. Pharmacol. 2006, 548, 167–173. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Gow, A.; Thom, S.R.; Kooy, N.W.; Royall, J.A.; Crow, J.P. Detection of Reactive Nitrogen Species using 2,7-Dichlorodihydrofluorescein and Dihydrorhodamine 123. In Methods in Enzymology—Nitric Oxide Part C—Biological and Antioxidant Activities; Abelson, J.N., Simon, M.I., Sies, H., Eds.; Academic Press: New York, NY, USA, 1999; Volume 301. [Google Scholar]
- Qin, Y.; Lu, M.; Gong, X. Dihydrorhodamine 123 is superior to 2,7-dichlorodihydrofluorescein diacetate and dihydrorhodamine 6G in detecting intracellular hydrogen peroxide in tumor cells. Cell Biol. Int. 2008, 32, 224–228. [Google Scholar] [CrossRef]
- Henderson, L.M.; Chappell, J.B. Dihydrorhodamine 123: A fluorescent probe for superoxide generation? Eur. J. Biochem. 1993, 217, 973–980. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Cultures of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Pippen, E.L.; Nonaka, M. A convenient method for synthesizing normal aliphatic 2,4-dienals. J. Org. Chem. 1958, 23, 1580–1582. [Google Scholar] [CrossRef]
- Fernandes, E.; Costa, D.; Toste, S.A.; Lima, J.; Reis, S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic. Biol. Med. 2004, 37, 1895–1905. [Google Scholar] [CrossRef]
- Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D. Nitric oxide production in tobacco leaf cells: A generalized stress response? Plant Cell Environ. 2003, 26, 1851–1862. [Google Scholar]
- Romano, G.; Costantini, M.; Buttino, I.; Ianora, A.; Palumbo, A. Nitric Oxide mediates the stress response induced by diatom aldehydes in the sea urchin Paracentrotus lividus. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Akaike, T.; Yoshida, M.; Miyamoto, Y.; Sato, K.; Kohno, M.; Sasamoto, K.; Miyazaki, K.; Ueda, S.; Maeda, H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor NO through a radical reaction. Biochemistry 1993, 32, 827–832. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and antihypertensive effects of Tempol and other nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Cataldo, M.L.; Conversano, F.; Brunet, C. Growth and photophysiological responses of two picoplanktonic Minutocellus species, strains RCC967 and RCC703 (Bacillariophyceae). Eur. J. Phycol. 2012, 47, 408–420. [Google Scholar] [CrossRef]
- Lucas, C.E. The ecological effects of external metabolites. Biol. Rev. Camb. Philos. Soc. 1947, 22, 270–295. [Google Scholar] [CrossRef]
- Lewis, W.M. Evolutionary interpretations of allelochemical interactions in phytoplankton algae. Am. Nat. 1986, 127, 184–194. [Google Scholar]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.; Bowler, C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Leflaive, J.; Ten-Hage, L. Chemical interactions in diatoms: Role of polyunsaturated aldehydes and precursors. New Phytol. 2009, 184, 794–805. [Google Scholar] [CrossRef]
- Vidoudez, C.; Pohnert, G. Growth phase-specific release of polyunsaturated aldehydes by the diatom Skeletonema marinoi. J. Plankton Res. 2008, 30, 1305–1313. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallina, A.A.; Brunet, C.; Palumbo, A.; Casotti, R. The Effect of Polyunsaturated Aldehydes on Skeletonema marinoi (Bacillariophyceae): The Involvement of Reactive Oxygen Species and Nitric Oxide. Mar. Drugs 2014, 12, 4165-4187. https://doi.org/10.3390/md12074165
Gallina AA, Brunet C, Palumbo A, Casotti R. The Effect of Polyunsaturated Aldehydes on Skeletonema marinoi (Bacillariophyceae): The Involvement of Reactive Oxygen Species and Nitric Oxide. Marine Drugs. 2014; 12(7):4165-4187. https://doi.org/10.3390/md12074165
Chicago/Turabian StyleGallina, Alessandra A., Christophe Brunet, Anna Palumbo, and Raffaella Casotti. 2014. "The Effect of Polyunsaturated Aldehydes on Skeletonema marinoi (Bacillariophyceae): The Involvement of Reactive Oxygen Species and Nitric Oxide" Marine Drugs 12, no. 7: 4165-4187. https://doi.org/10.3390/md12074165
APA StyleGallina, A. A., Brunet, C., Palumbo, A., & Casotti, R. (2014). The Effect of Polyunsaturated Aldehydes on Skeletonema marinoi (Bacillariophyceae): The Involvement of Reactive Oxygen Species and Nitric Oxide. Marine Drugs, 12(7), 4165-4187. https://doi.org/10.3390/md12074165