Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives
Abstract
:1. Introduction

2. New Compounds and Their Distribution 2001–2010
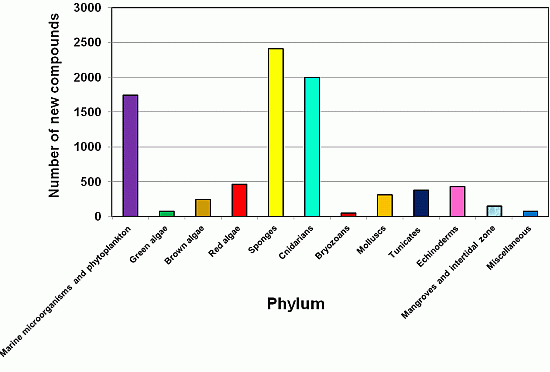
2.1. Yearly Distribution of Phyla that Produce Natural Products Discovered from 2001 to 2010

2.2. Sponges (Porifera) as a Source of New Natural Products and Drugs for the Future
2.3. The Distribution of New Marine Natural Products from Sponges

| Order | Number of Families | Number of Genera | Number of Species | Number of References |
|---|---|---|---|---|
| Agelasida | 9 | 9 | 21 | 24 |
| Astrophorida | 26 | 58 | 63 | 62 |
| Axinellida | 2 | 2 | 2 | 2 |
| Chondrosida | 1 | 1 | 1 | 1 |
| Choristida | 3 | 3 | 3 | 3 |
| Clathrinida | 5 | 5 | 7 | 7 |
| Dendroceratida | 7 | 10 | 11 | 13 |
| Dictyoceratida | 40 | 117 | 145 | 161 |
| Hadromerida | 24 | 31 | 32 | 33 |
| Halichondrida | 31 | 69 | 86 | 84 |
| Haplosclerida | 52 | 80 | 100 | 120 |
| Homosclerophorida | 10 | 20 | 39 | 50 |
| Leucosolenida | 1 | 1 | 1 | 1 |
| Lithistida | 14 | 20 | 23 | 32 |
| Lyssacinosida | 2 | 2 | 2 | 2 |
| Ocilosclerida | 1 | 1 | 1 | 2 |
| Poecilosclerida | 67 | 68 | 81 | 83 |
| Spirophorida | 4 | 4 | 4 | 5 |
| Unknown | 8 | 12 | 12 | 10 |
| Verongida | 27 | 29 | 37 | 46 |
| Total | 334 | 542 | 671 | 741 |

2.4. Distribution of New Compounds per Species from Different Orders
2.5. Symbiotic Relationships: Sponge Associated Microorganisms
2.6. The Distribution of Chemical Classes

2.7. The Distribution of Bioactive Compounds
| Orders of Sponges | Anti-Alzheimer’s | Antibacterial | Antituberculosis | Anticancer/Cytotoxicity | Antifungal | Anti-inflammatory | Antimalarial | Anti-HIV | Antiviral | Miscellaneous | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agelasida | 0 | 17 | 0 | 11 | 6 | 0 | 6 | 0 | 0 | 14 | 54 |
| Astrophorida | 0 | 8 | 6 | 97 | 7 | 0 | 1 | 5 | 3 | 22 | 149 |
| Axinellida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 8 |
| Chondrosida | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 6 |
| Choristida | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 12 | 24 |
| Clathrinida | 0 | 4 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Dendroceratida | 0 | 4 | 0 | 14 | 3 | 3 | 0 | 0 | 0 | 14 | 38 |
| Dictyoceratida | 0 | 38 | 3 | 182 | 11 | 2 | 1 | 5 | 0 | 90 | 332 |
| Hadromerida | 0 | 2 | 3 | 45 | 1 | 0 | 0 | 5 | 0 | 18 | 74 |
| Halichondrida | 1 | 18 | 4 | 99 | 16 | 1 | 2 | 1 | 0 | 31 | 173 |
| Haplosclerida | 8 | 15 | 2 | 100 | 20 | 0 | 7 | 4 | 0 | 73 | 229 |
| Homosclerophorida | 0 | 2 | 3 | 55 | 10 | 0 | 9 | 1 | 0 | 34 | 114 |
| Leucosolenida | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Lithistida | 0 | 5 | 2 | 38 | 9 | 2 | 0 | 16 | 0 | 16 | 88 |
| Lyssacinosida | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ocilosclerida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Poecilosclerida | 0 | 17 | 5 | 143 | 21 | 1 | 1 | 4 | 1 | 34 | 227 |
| Spirophorida | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Unknown | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 7 | 9 |
| Verongida | 0 | 14 | 0 | 17 | 5 | 0 | 0 | 0 | 0 | 34 | 70 |
| Total | 9 | 145 | 31 | 828 | 114 | 9 | 27 | 41 | 4 | 407 | 1615 |


| Organism | Order | Compound Name | Chemical Class | Special Feature/Activity | Source, Country, Year,/Depth | Reference |
|---|---|---|---|---|---|---|
| Sarcotragus sp. | Dictyoceratida | Sarcotragin A, & B | Trisnorsesterterpenoid lactam | Showed moderate cytotoxicity (LC50 207 μg/mL) toward the leukemia cell-line K562 | Seoguipo, Jaeju Island, Korea, 2001 | [146] |
| Polymastia tenax | Hadromerida | 5α,6α-epoxy-24R*-ethylcholest-8(14)-en-3β,7α-diol and 5α,6α-epoxy-24R*-ethylcholest-8-en-3β,7α-diol | Sterol | Exhibited significant cytotoxic activity vs. human lung carcinoma (A-549), human colon carcinomas (HT-29 and H-116), and human prostate carcinoma (PC-3) cell lines with the LC50 (μg/mL) value of 5–10, 1–5, 1–5, 0.5–1 and 1–5 | Punta de Betín, Bahía de Santa Marta, in the Colombian Caribbean, Colombia, 2002 | [147] |
| Crella spinulata | Poecilosclerida | Benzylthiocrellidone | Bis-dimedone thioether | First report of a natural product containing a dimedone moiety. No activity reported | Davies and Bowden Reefs Australia, 2002 | [148] |
| Ectyoplasia ferox | Poecilosclerida | Ectyoceramide | Galactofuranosylceramide (GSL) | The first example of a monohexofuranosylceramide and the first natural GSL with its first sugar in the furanose form. No activity reported | Island of Rum Cay, Bahamas, 2000 | [149] |
| Cribrochalina olemda | Haplosclerida | Kapakahine E | Peptide (cyclic) | Kapakahine E showed moderate cytotoxicity against P388 murine leukemia cells at IC50 of 5.0 μg/mL | Pohnpei, Micronesia, 2003 | [150] |
| Haliclona Viscosa | Haplosclerida | Viscosamine | Trimeric 3-alkyl pyridinium alkaloid | First trimeric 3-alkyl pyridinium compound from a marine environment. No activity reported | Coast of Blomstrandhalvøya, near Hansneset, Kongsfjorden, Arctic Ocean, 2003 | [151] |
| Phakellia fusca | Axinellida | Compound 1, 2, 3 | 5-Fluorouracil alkaloid | First report of fluorine containing natural products from a marine source. No activity reported | Yongxiong Island of the Xisha Islands, South China Sea, China 2003 | [152] |
| Agelas clathrodes | Agelasida | Clarhamnoside | Rhamnosylated R-Galactosylceramide | The first Rhamnosylated R-Galactosylceramide, a glycolipid containing an unusual l-rhamnose unit. No activity reported | Grand Bahamas Island (Sweetings Cay), Bahamas, 2004 | [153] |
| Psammocinia sp. | Dictyoceratida | Psymberin | Cytotoxin (distantly related to the Pederin family) | Several melanoma, breast, and colon cancer cell lines demonstrated high sensitivity (LC50 < 2.5 × 10−9 M) to psymberin, and all six leukemia cell lines proved comparably insensitive | Papua New Guinea, 2004 | [154] |
| Callyspongia abnormis | Haploscerida | Callynormine A | Cyclic Peptide | Represents a new class of heterodetic cyclic peptides (designated endiamino peptides). This compound possessing an α-amido-β-aminoacrylamide cyclization functionality | Shimoni reef, Kenya, 2004 | [155] |
| Axinella infundibula | Halichondrida | Axinelloside A | Lipopolysaccharide (Sulfated) | Axinelloside A, a complex polysulfated glycolipid, which strongly inhibited the activity of human telomerase with an IC50 value of 0.4 μM | Shikine-jima Island, the Izu Islands, Japan, 2005 | [156] |
| Theonella swinhoei | Lithistida | Plytheonamide A, B | Polypeptide | Showed cytotoxicity against P388 murine leukemia cells with IC50 values of 78 and 68 pg/mL, respectively. Linear polypeptides with unprecedented structural features | Hachijo-jima Island, Japan, 2005 | [157] |
| Neopetrosia sp. | Haplosclerida | Neopetrosiamide A, B | Peptide (diastereomeric tricyclic) | Active in inhibiting the amoeboid invasion by human tumor cells | Near Milne Bay, Papua New Guinea, 2005 | [158] |
| Prianos osiros | Haplosclerida | (3 R,3′R,5S)-3,3′,5,19′-tetrahydroxy-7′,8′-didehydro-γ,ε-carotene-8-one | Acetylenic carotenoid | Contains an unusual cytotoxic carotenoid | Pohnpei, Micronesia, 2005 | [159] |
| Ircinia sp. | Dictyoceratida | Irciniasulfonic acid B | Fatty acid derivative (taurine conjugated) | Reversed the multi-drug resistance to vincristine in KB/VJ300 cells at the concentration of 100 μM | Tsuzumi Island, Fukuoka Prefecture, Japan, 2006 | [160] |
| Suberites japonicus | Hadromerida | Seragamide A–F | Depsipeptide (actin targeting) | Caused multinuclei formation in cells at 0.01–0.02 μg/mL | Seragaki, Okinawa, Japan, 2006 | [161] |
| Theonella swinhoei | Lithistida | Hurghadolide A | Macrolide | Caused disruption of the actin cytoskeleton at concentrations of 7.3 nM. Active against Candida albicans (MIC 31.3 μg/mL) | Red Sea, Egypt, 2006 | [89] |
| Theonella swinhoei | Lithistida | Swinholide I | Macrolide | as above | Red Sea, Egypt, 2006 | [89] |
| Coelocarteria cfr. singaporensis | Poecilosclerida | Coelodiol and Coelic acid | Diterpene (ent-isocopalane) | Inhibit the in vitro growth of MKN-45 cell line (human gastric adenocarcinoma) at 20 and 40 μg/mL respectively | Bunaken, Marine Park (North Sulawesi), Indonesia, 2006 | [162] |
| Lendenfeldia sp. | Dictyoceratida | ( S)-2,2′-Dimethoxy-1,1′-binaphthyl-5,5′,6,6′-tetraol | Naphthalene dimer | Significantly inhibited both hypoxia-induced (IC50 values 4.3 µM) and iron chelator (1, 10-phenanthroline)-induced HIF-1 activation in T47D breast tumor cells. This compound inhibited HIF-1 activation at concentrations that were significantly lower than those that suppressed tumor cell viability | Collected at 2 m depth on May 22, 1993 (sample C011337), from a sea grass bed, Indonesia, 2007 | [163] |
| Erylus formosus | Astrophorida | Eryloside F1–F4 | Triterpene glycoside | At a concentration of 100 μg/mL were found to activate Ca2 influx into mouse spleenocytes. biosides having aglycons related to penasterol with additional oxidation patterns in their side chains | Puerto Morelos (the Caribbean Sea), Mexico 2007 | [164] |
| Erylus formosus | Astrophorida | Eryloside M–Q | Triterpene glycoside | As above, contain new variants of carbohydrate chains with three, four and six sugar units. Contain 14-carboxy-24-methylenelanost-8(9)-en-3β-ol | Puerto Morelos (the Caribbean Sea), Mexico, 2007 | [164] |
| Cacospongia mycofijiensis | Dictyoceratida | CTP-431 | Thiopyrone | Showed only mild cytotoxicity (IC50: 18 μM) against human colon carcinoma HCT-116. This compound has no previous precedent in natural products chemistry. Its structure including absolute configuration as 8R,9R,10S,13S | Beqa Lagoon, Fiji, 2008 | [165] |
| Homophymia sp. | Lithistida | Homophymine A | Cyclodepsipeptide | Exhibited cytoprotective activity against HIV-1 infection with a IC50 of 75 nM | Coast of New Caledonia, 2008 | [166] |
| Ianthella sp. | Verongida | Petrosterol-3,6-dione and 5α,6α-epoxy-petrosterol | C29 sterol | Showed growth-inhibitory effects with IC50 values of 8.4, 19.9, 17.8, 16.2 and 22.1 μM against lung (A549), colon (HT-29), breast (MCF-7), ovary (SK-OV-3), and two types of leukemia (HL-60 and U937) human cancer cell lines | Namyet Island, Khanh Hoa province, Vietnam, 2009 | [167] |
| Topsentia sp. | Halichondrida | Geodisterol-3- O-sulfite and 29-demethylgeodisterol-3-O-sulfite | Sterol (sulphated) | Reverses efflux pump mediated fluconazole resistance. Also enhances fluconazole activity in a Saccharomyces cerevisiae strain overexpressing the Candida albicans efflux pump MDR1, as well as in a fluconazole-resistant Candida albicans clinical isolate known to overexpress MDR1 | Chuuk, Micronesia, 2009 | [168] |
| Spongia (Heterofibria) sp. | Dictyoceratida | Heterofibrin A1–A3 and B1–B3 | Fatty acid | Possess a diyne-ene moiety, while the monolactyl and dilactyl moiety featured in selected heterofibrins is unprecedented in the natural products literature. Inhibited lipid droplet formation in A431 fibroblast cells (up to 60% at 10 μM) | Great Australian Bight, Australia, 2010 | [169] |
| Xestospongia sp. | Haplosclerida | Xestosaprol F–M | Xestosaprol (pentacyclic compound) | Showed moderate inhibition of the aspartic protease BACE1 (memapsin-2), which has a central role in the etiology of Alzheimer’s disease with the IC50 value of 135 ± 11 μM. First examples of a monooxygenated A-ring | Coral reef at Sangalaki, Indonesia, 2010 | [170] |
| Theonella swinhoei | Lithistida | Paltolides A–C | Peptides (Anabaenopeptin like) | Closely related to a group of anabaenopeptins that are submicromolar inhibitors of carboxypeptidase U with greater than 50 fold selectivity over other carboxypeptidases | Uchelbeluu Reef, Palau, 2010 | [171] |
| Neopetrosia proxima | Haplosclerida | Neopetrosiamine A | Alkaloid (tetracyclic bis-piperidine) | Exhibited strong inhibitory activity against MALME-3M melanoma cancer, CCRF-CEM leukemia, and MCF7 breast cancer with IC50 values of 1.5, 2.0, and 3.5 μM, respectively. In vitro activity vs. pathogenic strain of Mycobacterium tuberculosis (H37Rv) and Plasmodium falciparum | Mona Island, Puerto Rico, 2010 | [172] |
| Iotrochota baculifera | Poecilosclerida | Baculiferins A–O | O-sulfated pyrrole alkaloids | Baculiferins C, E–H, and K–N (4, 6–9, 12–15) are potent inhibitors of HIV-1 IIIB virus in both MT4 and MAGI cells. Additionally could bind to the HIV-1 target proteins Vif, APOBEC3G, and gp41 | Inner coral reef, Hainan Island, China, 2010 | [173] |
2.8. Distribution of New Compounds Based on Country/Geographical Area


3. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contribution
Conflicts of Interest
References
- Bergmann, W.; Feeney, R.J. Contributions to the study of marine products. XXXII. The nucleosides of sponges I. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges 1. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Carte, B.K. Biomedical potential of marine natural products. Bioscience 1996, 46, 271–287. [Google Scholar] [CrossRef]
- Burkholder, P.R.; Pfister, R.M.; Leitz, F.H. Production of a pyrrole antibiotic by a marine bacterium. Appl. Microbiol. 1966, 14, 649–653. [Google Scholar]
- Proksch, P.; Edrada, R.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar] [CrossRef]
- Weinheimer, A.J.; Spraggins, R.L. The occurrence of two new prostaglandin derivatives (15-epi-PGA and its acetate, methyl ester) in the Gorgonian Plexaura homomalla chemistry of Coelenterates. XV. Tetrahedron Lett. 1969, 10, 5185–5188. [Google Scholar] [CrossRef]
- Ireland, C.M.; Copp, B.R.; Foster, M.P.; McDonald, L.A.; Radisky, D.C.; Swersey, J.C. Biomedical potential of marine natural products. In Pharmaceutical and Bioactive Natural Products; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–43. [Google Scholar]
- Gordon, E.M.; Barrett, R.W.; Dower, W.J.; Fodor, S.P.; Gallop, M.A. Applications of combinatorial technologies to drug discovery. 2. Combinatorial organic synthesis, library screening strategies, and future directions. J. Med. Chem. 1994, 37, 1385–1401. [Google Scholar]
- Alonso, D.; Khalil, Z.; Satkunanthan, N.; Livett, B. Drugs from the sea: Conotoxins as drug leads for neuropathic pain and other neurological conditions. Mini Rev. Med. Chem. 2003, 3, 785–787. [Google Scholar] [CrossRef]
- Hu, G.P.; Yuan, J.; Sun, L.; She, Z.G.; Wu, J.H.; Lan, X.J.; Zhu, X.; Lin, Y.C.; Chen, S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2000, 17, 7–55. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Laport, M.; Santos, O.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharma. Biotechnol. 2009, 10, 86–105. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H. G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Wijffels, R.H. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol. 2008, 26, 26–31. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting? PLoS One 2012, 7, e30580. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H. G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2003, 20, 1–48. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H. G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.-P.; Munro, M.H. G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef]
- Hooper, J.N. Sponguide. Guide to Sponge Collection and Identification; Queensland Museum: Queensland, Australia, 2000. [Google Scholar]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2006, 23, 153–180. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–624. [Google Scholar]
- Lee, Y.K.; Lee, J.-H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Jensen, P.R.; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological perspectives. Ann. Rev. Microbiol. 1994, 48, 559–584. [Google Scholar]
- Bernan, V.; Greenstein, M.; Maiese, W. Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol. 1997, 43, 57–90. [Google Scholar] [CrossRef]
- Haygood, M.G.; Schmidt, E.W.; Davidson, S.K.; Faulkner, D.J. Microbial symbionts of marine invertebrates: Opportunities for microbial biotechnology. J. Mol. Microbiol. Biotechnol. 1999, 1, 33–43. [Google Scholar]
- Osinga, R.; Armstrong, E.; Burgess, J.G.; Hoffmann, F.; Reitner, J.; Schumann-Kindel, G. Sponge—Microbe associations and their importance for sponge bioprocess engineering. Hydrobiologia 2001, 461, 55–62. [Google Scholar] [CrossRef]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef]
- Li, C.-W.; Chen, J.-Y.; Hua, T.-E. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar] [CrossRef]
- Hentschel, U.; Hopke, J.; Horn, M.; Friedrich, A.B.; Wagner, M.; Hacker, J.; Moore, B.S. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 2002, 68, 4431–4440. [Google Scholar] [CrossRef]
- Selvin, J.; Shanmugha Priya, S.; Seghal Kiran, G.; Thangavelu, T.; Sapna Bai, N. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol. Res. 2009, 164, 352–363. [Google Scholar] [CrossRef]
- Wilkinson, C.; Fay, P. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature 1979, 279, 527–529. [Google Scholar] [CrossRef]
- Diaz, M.C.; Rutzler, K. Sponges: An essential component of Caribbean coral reefs. Bull. Mar. Sci. 2001, 69, 535–546. [Google Scholar]
- Thoms, C.; Horn, M.; Wagner, M.; Hentschel, U.; Proksch, P. Monitoring microbial diversity and natural product profiles of the sponge Aplysina cavernicola following transplantation. Mar. Biol. 2003, 142, 685–692. [Google Scholar]
- Thoms, C.; Schupp, P. Biotechnological potential of marine sponges and their associated bacteria as producers of new pharmaceuticals (Part II). J. Int. Biotechnol. Law 2005, 2, 257–264. [Google Scholar]
- Proksch, P. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon 1994, 32, 639–655. [Google Scholar] [CrossRef]
- Pawlik, J.R.; McFall, G.; Zea, S. Does the odor from sponges of the genus Ircinia protect them from fish predators? J. Chem. Ecol. 2002, 28, 1103–1115. [Google Scholar] [CrossRef]
- Mahon, A.R.; Amsler, C.D.; McClintock, J.B.; Amsler, M.O.; Baker, B.J. Tissue-specific palatability and chemical defenses against macropredators and pathogens in the common articulate brachiopod Liothyrella uva from the Antarct. Penins. J. Exp. Mar. Biol. Ecol. 2003, 290, 197–210. [Google Scholar] [CrossRef]
- Paul, V.; Cruz-Rivera, E.; Thacker, R. Chemical mediation of macroalgal-herbivore interactions: Ecological and evolutionary perspectives. In Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001; pp. 227–265. [Google Scholar]
- Unson, M.D.; Holland, N.D.; Faulkner, D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 1994, 119, 1–11. [Google Scholar] [CrossRef]
- Vacelet, J.; Vacelet, E.; Gaino, E.; Gallissian, M. Bacterial attack of spongin skeleton during the 1986–1990 Mediterranean sponge disease. In Sponges in Time and Space; Balkema: Rotterdam, The Netherlands, 1994; pp. 355–362. [Google Scholar]
- Boehm, M.; Hentschel, U.; Friedrich, A.; Fieseler, L.; Steffen, R.; Gamulin, V.; Mueller, I.; Müller, W. Molecular response of the sponge Suberites domuncula to bacterial infection. Mar. Biol. 2001, 139, 1037–1045. [Google Scholar] [CrossRef]
- Maldonado, M.; Sánchez-Tocino, L.; Navarro, C. Recurrent disease outbreaks in corneous demosponges of the genus Ircinia: Epidemic incidence and defense mechanisms. Mar. Biol. 2010, 157, 1577–1590. [Google Scholar] [CrossRef]
- Selvin, J.; Ninawe, A.; Seghal Kiran, G.; Lipton, A. Sponge-microbial interactions: Ecological implications and bioprospecting avenues. Crit. Rev. Microbiol. 2010, 36, 82–90. [Google Scholar] [CrossRef]
- Thakur, N.L.; Müller, W.E. Biotechnological potential of marine sponges. Curr. Sci. 2004, 86, 1506–1512. [Google Scholar]
- Tilvi, S.; Rodrigues, C.; Naik, C.; Parameswaran, P.; Wahidhulla, S. New bromotyrosine alkaloids from the marine sponge Psammaplysilla purpurea. Tetrahedron 2004, 60, 10207–10215. [Google Scholar] [CrossRef]
- Abad, M.; Bedoya, L.; Bermejo, P. Marine compounds and their antimicrobial activities. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011. [Google Scholar]
- Van Soest, R.; Boury-Esnault, N.; Hooper, J.; Rützler, K.; de Voogd, N.; Alvarez de Glasby, B.; Hajdu, E.; Pisera, A.; Manconi, R.; Schoenberg, C. World Porifera Database. The World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/porifera (accessed on 25 October 2012).
- Appeltans, W.; Decock, W.; Vanhoorne, B.; Hernandez, F.; Bouchet, P.; Boxshall, G.; Fauchald, K.; Gordon, D.P.; Poore, G.C.B.; van Soest, R.; et al. The World Register of Marine Species. In Proceedings of the future of the 21st century ocean: Marine Sciences and European Research Infrastructures, Brest, France, 28 June–1 July 2011; p. 30.
- Ausubel, J.; Trew Christ, D.; Waggoner, P. First Census of Marine Life 2010: Highlights of A Decade of Discovery. Available online: http://www.coml.org/pressreleases/census2010/PDF/Highlights-2010-Report-Low-Res.pdf (accessed on 11 January 2014).
- Valerie, P.; Raphael, R.-W. Marine chemical ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef]
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1998, 15, 113–158. [Google Scholar] [CrossRef]
- Crews, P.; Gerwick, W.; Schmitz, F.; France, D.; Bair, K.; Wright, A.; Hallock, Y. Molecular approaches to discover marine natural product anticancer leads-an update from a drug discovery group collaboration. Pharm. Biol. 2003, 41, 39–52. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef]
- Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural sources as potential anti-cancer agents: A review. Int. J. Phytomed. 2011, 3, 9–26. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Thakur, A.N.; Thakur, N.L.; Indap, M.M.; Pandit, R.A.; Datar, V.V.; Müller, W.E. Antiangiogenic, antimicrobial, and cytotoxic potential of sponge-associated bacteria. Mar. Biotechnol. 2005, 7, 245–252. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef]
- Petit, K.; Biard, J.-F. Marine natural products and related compounds as anticancer agents: An overview of their clinical status. Anti-Cancer Agents Med. Chem. 2013, 13, 603–631. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Chin, Y.-W.; Swanson, S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Dev. 2009, 12, 189–196. [Google Scholar]
- Duckworth, A. Farming sponges to supply bioactive metabolites and bath sponges: A review. Mar. Biotechnol. 2009, 11, 669–679. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Böhm, M.; Batel, R.; de Rosa, S.; Tommonaro, G.; Müller, I.M.; Schröder, H.C. Application of cell culture for the production of bioactive compounds from sponges: Synthesis of Avarol by primmorphs from Dysidea avara. J. Nat. Prod. 2000, 63, 1077–1081. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Zhang, W.; Yu, X.; Jin, M. Primmorphs from archaeocytes-dominant cell population of the sponge Hymeniacidon perleve: Improved cell proliferation and spiculogenesis. Biotechnol. Bioeng. 2003, 84, 583–590. [Google Scholar] [CrossRef]
- Müller, W.E.; Grebenjuk, V.A.; le Pennec, G.; Schröder, H.-C.; Brümmer, F.; Hentschel, U.; Müller, I.M.; Breter, H.-J. Sustainable production of bioactive compounds by sponges—Cell culture and gene cluster approach: A review. Mar. Biotechnol. 2004, 6, 105–117. [Google Scholar]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Towards commercial production of sponge medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar] [CrossRef]
- Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.; Heycke, N.; Schmitt, S. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62. [Google Scholar] [CrossRef]
- Jaspars, M.; Challis, G. Microbiology: A talented genus. Nature 2014, 506, 38–39. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004, 21, 519–538. [Google Scholar] [CrossRef]
- Van Soest, R.W.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N. Global diversity of sponges (Porifera). PLoS One 2012, 7, e35105. [Google Scholar]
- Kalaitzis, J.A. 2005. Chemical Investigations of Australian Marine Sponges. Available online: https://www120.secure.griffith.edu.au/rch/file/786c6d3d-925a-dea0-3864-2db686e40950/1/02Chapter1.pdf (accessed on 29 April 2014).
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N. Nonribosomal peptides from marine sponges. Curr. Org. Chem. 2003, 7, 945–966. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Kato, Y.; Hirota, H. Aurantosides A and B: Cytotoxic tetramic acid glycosides from the marine sponge Theonella sp. J. Am. Chem. Soc. 1991, 113, 9690–9692. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Structure of swinholide-a, a new macrolide from the marine sponge Theonella swinhoei. Tetrahedron Lett. 1985, 26, 511–514. [Google Scholar] [CrossRef]
- Youssef, D.T.; Mooberry, S.L. Hurghadolide A and Swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Webster, N.S.; Blackall, L.L. What do we really know about sponge-microbial symbioses? Int. Soc. Micobial. Ecol. J. 2009, 3, 1–3. [Google Scholar]
- Webster, N.S.; Taylor, M.W. Marine sponges and their microbial symbionts: Love and other relationships. Environ. Microbiol. 2012, 14, 335–346. [Google Scholar] [CrossRef]
- Mohamed, N.M.; Rao, V.; Hamann, M.T.; Kelly, M.; Hill, R.T. Monitoring bacterial diversity of the marine sponge Ircinia strobilina upon transfer into aquaculture. Appl. Environ. Microbiol. 2008, 74, 4133–4143. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Net primary productivity in coral reef sponges. Science 1983, 219, 410–412. [Google Scholar]
- Corredor, J.E.; Wilkinson, C.R.; Vicente, V.P.; Morell, J.M.; Otero, E. Nitrate release by Caribbean reef sponges. Limnol. Oceanogr. 1988, 33, 114–120. [Google Scholar] [CrossRef]
- Diaz, M.C.; Ward, B.B. Sponge-mediated nitrification in tropical benthic communities. Mar. Ecol. Prog. Ser. 1997, 156, 97–107. [Google Scholar] [CrossRef]
- Shieh, W.Y.; Lin, Y.M. Association of heterotrophic nitrogen-fixing bacteria with a marine sponge of Halichondria sp. Bull. Mar. Sci. 1994, 54, 557–564. [Google Scholar]
- Wilkinson, C.C.; Summons, R.R.; Evans, E. Nitrogen fixation in symbiotic marine sponges: Ecological significance and difficulties in detection. Mem. Qld. Mus. 1999, 44, 667–673. [Google Scholar]
- Hoffmann, F.; Larsen, O.; Thiel, V.; Rapp, H.T.; Pape, T.; Michaelis, W.; Reitner, J. An anaerobic world in sponges. Geomicrobiol. J. 2005, 22, 1–10. [Google Scholar] [CrossRef]
- Hentschel, U.; Fieseler, L.; Wehrl, M.; Gernert, C.; Steinert, M.; Hacker, J.; Horn, M. Microbial diversity of marine sponges. In Sponges (Porifera); Springer: Berlin/Heidelberg, Germany, 2003; pp. 59–88. [Google Scholar]
- Kennedy, J.; Baker, P.; Piper, C.; Cotter, P.D.; Walsh, M.; Mooij, M.J.; Bourke, M.B.; Rea, M.C.; O’Connor, P.M.; Ross, R.P. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar. Biotechnol. 2009, 11, 384–396. [Google Scholar] [CrossRef]
- Rahman, H.; Austin, B.; Mitchell, W.J.; Morris, P.C.; Jamieson, D.J.; Adams, D.R.; Spragg, A.M.; Schweizer, M. Novel anti-infective compounds from marine bacteria. Mar. Drugs 2010, 8, 498–518. [Google Scholar] [CrossRef]
- Lu, X.; Cao, X.; Liu, X.; Jiao, B. Marine microbes-derived anti-bacterial agents. Mini Rev. Med. Chem. 2010, 10, 1077–1090. [Google Scholar] [CrossRef]
- Dudler, R.; Eberl, L. Interactions between bacteria and eukaryotes via small molecules. Curr. Opin. Biotechnol. 2006, 17, 268–273. [Google Scholar]
- Schmidt, E.W. Trading molecules and tracking targets in symbiotic interactions. Nat. Chem. Biol. 2008, 4, 466–473. [Google Scholar]
- Hu, J.-F.; Hamann, M.T.; Hill, R.; Kelly, M. The manzamine alkaloids. Alkaloids Chem. Biol. 2003, 60, 207–285. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Sakai, R.; Rinehart, K.L. Crambescidins: New antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem. 1991, 56, 5712–5715. [Google Scholar] [CrossRef]
- Berlinck, R.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef]
- Croué, J.; West, N.J.; Escande, M.-L.; Intertaglia, L.; Lebaron, P.; Suzuki, M.T. A single betaproteobacterium dominates the microbial community of the crambescidine-containing sponge Crambe crambe. Sci. Rep. 2013, 3, 1–8. [Google Scholar]
- Sladic, D.; Gasic, M.J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules 2006, 11, 1–33. [Google Scholar]
- Assmann, M.; Lichte, E.; Pawlik, J.R.; Köck, M. Chemical defenses of the Caribbean sponges Agelas wiedenmayeri and Agelas conifera. Mar. Ecol. Prog. Ser. 2000, 207, 255–262. [Google Scholar]
- Neeman, I.; Fishelson, L.; Kashman, Y. Isolation of a new toxin from the sponge Latrunculia magnifica in the Gulf of Aquaba (Red Sea). Mar. Biol. 1975, 30, 293–296. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Davies-Coleman, M.T. Anti-inflammatory metabolites from marine sponges. Chem. Soc. Rev. 2005, 34, 355–365. [Google Scholar] [CrossRef]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Blunt, J.W.; Munro, M.H.G.; Battershill, C.N.; Page, M.J. The whys and whats of sponge chemistry: Why chemists extract sponges and what problems does this cause? In Sponge Sciences. Multidiscipinary Perspectives; Watanabe, Y., Fusetani, N., Eds.; Springer-Verlag: Tokyo, Japan, 1998; pp. 353–364. [Google Scholar]
- Sacristán-Soriano, O.; Banaigs, B.; Casamayor, E.O.; Becerro, M.A. Exploring the links between natural products and bacterial assemblages in the sponge Aplysina aerophoba. Appl. Environ. Microbiol. 2011, 77, 862–870. [Google Scholar] [CrossRef]
- Rinehart, K.L. Secondary metabolites from marine organisms. Ciba Found. Symp. 1992, 171, 236–249. [Google Scholar]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Mohammed, R.; Peng, J.; Kelly, M.; Hamann, M.T. Cyclic heptapeptides from the Jamaican sponge Stylissa caribica. J. Nat. Prod. 2006, 69, 1739–1744. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Konosu, S. Bioactive marine metabolites, IV. Isolation and the amino acid composition of discodermin A, an antimicrobial peptide, from the marine sponge Discodermia kiiensis. J. Nat. Prod. 1985, 48, 236–241. [Google Scholar] [CrossRef]
- Otero-González, A.J.; Magalhães, B.S.; Garcia-Villarino, M.; López-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013. [Google Scholar] [CrossRef]
- Cooper, E.L. Comparative immunology. Integr. Comp. Biol. 2003, 43, 278–280. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and cytotoxic compounds from marine organisms. In Pharmaceutical and Bioactive Natural Products; Springer: Berlin/Heidelberg, Germany, 1993; pp. 197–308. [Google Scholar]
- Zheng, L.; Yan, X.; Han, X.; Chen, H.; Lin, W.; Lee, F.S.; Wang, X. Identification of norharman as the cytotoxic compound produced by the sponge (Hymeniacidon perleve)-associated marine bacterium Pseudoalteromonas piscicida and its apoptotic effect on cancer cells. Biotechnol. Appl. Biochem. 2006, 44, 135–142. [Google Scholar] [CrossRef]
- Folmer, F.; Jaspars, M.; Dicato, M.; Diederich, M. Marine cytotoxins: Callers for the various dances of death. Gastroenterol. Hepatol. Bed Bench 2009, 2, S34–S50. [Google Scholar]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.-O.; Cho, H.Y.; Jung, J.H. Bisindole alkaloids of the topsentin and hamacanthin classes from a marine sponge Spongosorites sp. J. Nat. Prod. 2007, 70, 2–8. [Google Scholar] [CrossRef]
- Luo, X.; Li, F.; Hong, J.; Lee, C.-O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic oxylipins from a marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 567–571. [Google Scholar] [CrossRef]
- Mansoor, T.A.; Lee, Y.M.; Hong, J.; Lee, C.-O.; Im, K.S.; Jung, J.H. 5,6:8,9-Diepoxy and other cytotoxic sterols from the marine sponge Homaxinella sp. J. Nat. Prod. 2006, 69, 131–134. [Google Scholar] [CrossRef]
- Nagle, D.G.; Zhou, Y.-D.; Mora, F.D.; Mohammed, K.A.; Kim, Y.-P. Mechanism targeted discovery of antitumor marine natural products. Curr. Med. Chem. 2004, 11, 1725. [Google Scholar] [CrossRef]
- Aoki, S.; Cho, S.-H.; Ono, M.; Kuwano, T.; Nakao, S.; Kuwano, M.; Nakagawa, S.; Gao, J.-Q.; Mayumi, T.; Shibuya, M. Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anti-Cancer Drugs 2006, 17, 269–278. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, M.; Joshi, P.; Rawat, D.S. Clinical status of anti-cancer agents derived from marine sources. Anti-Cancer Agents Med. Chem. 2008, 8, 603–617. [Google Scholar] [CrossRef]
- Gong, H.; Zuliani, P.; Komuravelli, A.; Faeder, J.R.; Clarke, E.M. Analysis and verification of the HMGB1 signaling pathway. BMC Bioinform. 2010, 11, S10. [Google Scholar]
- Essack, M.; Bajic, V.B.; Archer, J.A. Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar. Drugs 2011, 9, 1580–1606. [Google Scholar] [CrossRef]
- Valeriote, F.A.; Tenney, K.; Pietraszkiewicz, H.; Edelstein, M.; Johnson, T.A.; Amagata, T.; Crews, P. Discovery and development of anticancer agents from marine sponges: Perspectives based on a chemistry-experimental therapeutics collaborative program. J. Exp. Ther. Oncol. 2012, 10, 119–134. [Google Scholar]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; Dilip de Silva, E.; Lassota, P.; Allen, T.M. Papuamides AD, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Qureshi, A.; Faulkner, D.J. Haplosamates A and B: New steroidal sulfamate esters from two haplosclerid sponges. Tetrahedron 1999, 55, 8323–8330. [Google Scholar] [CrossRef]
- Müller, W.E.; Sobel, C.; Diehl-Seifert, B.; Maidhof, A.; Schröder, H.C. Influence of the antileukemic and anti-human immunodeficiency virus agent avarol on selected immune responses in vitro and in vivo. Biochem. Pharmacol. 1987, 36, 1489–1494. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Yang, B.; Lin, X.; Yang, X.-W.; Liu, Y. Marine natural products with anti-HIV activities in the last decade. Curr. Med. Chem. 2013, 20, 953–973. [Google Scholar]
- Aoki, S.; Watanabe, Y.; Sanagawa, M.; Setiawan, A.; Kotoku, N.; Kobayashi, M. Cortistatins A, B, C, and D, anti-angiogenic steroidal alkaloids, from the marine sponge Corticium simplex. J. Am. Chem. Soc. 2006, 128, 3148–3149. [Google Scholar]
- Aoki, S.; Watanabe, Y.; Tanabe, D.; Arai, M.; Suna, H.; Miyamoto, K.; Tsujibo, H.; Tsujikawa, K.; Yamamoto, H.; Kobayashi, M. Structure-activity relationship and biological property of cortistatins, anti-angiogenic spongean steroidal alkaloids. Bioorg. Med. Chem. 2007, 15, 6758–6762. [Google Scholar] [CrossRef]
- Mousseau, G.; Clementz, M.A.; Bakeman, W.N.; Nagarsheth, N.; Cameron, M.; Shi, J.; Baran, P.; Fromentin, R.; Chomont, N.; Valente, S.T. An analog of the natural steroidal alkaloid cortistatin a potently suppresses tat-dependent hiv transcription. Cell Host Microbe 2012, 12, 97–108. [Google Scholar] [CrossRef]
- Andavan, G.S.B.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef]
- Shin, J.; Rho, J.R.; Seo, Y.; Lee, H.S.; Cho, K.W.; Sim, C.J. Sarcotragins A and B, new sesterterpenoid alkaloids from the sponge Sarcotragus sp. Tetrahedron Lett. 2001, 42, 3005–3007. [Google Scholar]
- Santafé, G.; Paz, V.; Rodríguez, J.; Jiménez, C. Novel cytotoxic oxygenated C29 Sterols from the Colombian marine sponge Polymastia tenax. J. Nat. Prod. 2002, 65, 1161–1164. [Google Scholar] [CrossRef]
- Pattenden, G.; Wickramasinghe, W.A.; Bandaranayake, W.M. Benzylthiocrellidone, a novel thioether with strong UV A and B absorption from the Great Barrier Reef sponge Crella spinulata, (Poecilosclerida: Crellidae). Trends Comp. Biochem. Physiol. 2002, 9, 205–216. [Google Scholar]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Ectyoceramide, the first natural hexofuranosylceramide from the marine sponge Ectyoplasia ferox. Eur. J. Org. Chem. 2003, 2003, 1433–1437. [Google Scholar]
- Nakao, Y.; Kuo, J.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. More Kapakahines from the marine Sponge Cribrochalina olemda. Org. Lett. 2003, 5, 1387–1390. [Google Scholar] [CrossRef]
- Volk, C.A.; Kǒck, M. Viscosamine: The first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org. Lett. 2003, 5, 3567–3569. [Google Scholar] [CrossRef]
- Xu, X.-H.; Yao, G.-M.; Li, Y.-M.; Lu, J.-H.; Lin, C.-J.; Wang, X.; Kong, C.-H. 5-Fluorouracil derivatives from the sponge Phakellia fusca. J. Nat. Prod. 2003, 66, 285–288. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Imperatore, C.; Mangoni, A. Glycolipids from sponges. 13.1 Clarhamnoside, the first rhamnosylated α-galactosylceramide from Agelas clathrodes. Improving spectral strategies for glycoconjugate structure determination. J. Org. Chem. 2004, 69, 1174–1179. [Google Scholar]
- Cichewicz, R.H.; Valeriote, F.A.; Crews, P. Psymberin, a potent sponge-derived cytotoxin from Psammocinia distantly related to the pederin family. Org. Lett. 2004, 6, 1951–1954. [Google Scholar] [CrossRef]
- Berer, N.; Rudi, A.; Goldberg, I.; Benayahu, Y.; Kashman, Y. Callynormine A, a new marine cyclic peptide of a novel class. Org. Lett. 2004, 6, 2543–2545. [Google Scholar] [CrossRef]
- Warabi, K.; Hamada, T.; Nakao, Y.; Matsunaga, S.; Hirota, H.; van Soest, R.W.M.; Fusetani, N. Axinelloside A, an unprecedented highly sulfated lipopolysaccharide inhibiting telomerase, from the marine sponge, Axinella infundibula. J. Am. Chem. Soc. 2005, 127, 13262–13270. [Google Scholar]
- Hamada, T.; Matsunaga, S.; Yano, G.; Fusetani, N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. Soc. 2005, 127, 110–118. [Google Scholar]
- Williams, D.E.; Austin, P.; Diaz-Marrero, A.R.; Soest, R.V.; Matainaho, T.; Roskelley, C.D.; Roberge, M.; Andersen, R.J. Neopetrosiamides, peptides from the marine sponge Neopetrosia sp. that inhibit amoeboid invasion by human tumor cells. Org. Lett. 2005, 7, 4173–4176. [Google Scholar] [CrossRef]
- Rogers, E.W.; Molinski, T.F. A cytotoxic carotenoid from the marine sponge Prianos osiros. J. Nat. Prod. 2005, 68, 450–452. [Google Scholar] [CrossRef]
- Emura, C.; Higuchi, R.; Miyamoto, T. Irciniasulfonic acid B, a novel taurine conjugated fatty acid derivative from a Japanese marine sponge, Ircinia sp. Tetrahedron 2006, 62, 5682–5685. [Google Scholar] [CrossRef]
- Tanaka, C.; Tanaka, J.; Bolland, R.F.; Marriott, G.; Higa, T. Seragamides A–F, new actin-targeting depsipeptides from the sponge Suberites japonicus Thiele. Tetrahedron 2006, 62, 3536–3542. [Google Scholar] [CrossRef]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Bavestrello, G.; Bonelli, P.; Calcinai, B. Coelodiol and coeloic acid, ent-isocopalane diterpenes from the Indonesian sponge Coelocarteria cfr. singaporensis. Tetrahedron Lett. 2006, 47, 2197–2200. [Google Scholar]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Cytotoxic metabolites from an Indonesian sponge Lendenfeldia sp. J. Nat. Prod. 2007, 70, 1824–1826. [Google Scholar] [CrossRef]
- Antonov, A.S.; Kalinovsky, A.I.; Stonik, V.A.; Afiyatullov, S.S.; Aminin, D.L.; Dmitrenok, P.S.; Mollo, E.; Cimino, G. Isolation and structures of Erylosides from the Carribean sponge Erylus formosus. J. Nat. Prod. 2007, 70, 169–178. [Google Scholar] [CrossRef]
- Johnson, T.A.; Amagata, T.; Oliver, A.G.; Tenney, K.; Valeriote, F.A.; Crews, P. The unexpected isolation of CTP-431, a novel thiopyrone from the sponge Cacospongia mycofijiensis. J. Org. Chem. 2008, 73, 7255–7259. [Google Scholar]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.C.; D’Auria, M.V.; Jepsen, T.; Petek, S.; Adeline, M.-T.R.S.; Laprévôte, O. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar]
- Tung, N.H.; Minh, C.V.; Ha, T.T.; Kiem, P.V.; Huong, H.T.; Dat, N.T.; Nhiem, N.X.; Tai, B.H.; Hyun, J.-H.; Kang, H.-K.; et al. C29 sterols with a cyclopropane ring at C-25 and 26 from the Vietnamese marine sponge Ianthella sp. and their anticancer properties. Bioorg. Med. Chem. Lett. 2009, 19, 4584–4588. [Google Scholar] [CrossRef]
- DiGirolamo, J.A.; Li, X.-C.; Jacob, M.R.; Clark, A.M.; Ferreira, D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J. Nat. Prod. 2009, 72, 1524–1528. [Google Scholar] [CrossRef]
- Salim, A.A.; Rae, J.; Fontaine, F.; Conte, M.M.; Khalil, Z.; Martin, S.; Parton, R.G.; Capon, R.J. Heterofibrins: Inhibitors of lipid droplet formation from a deep-water southern Australian marine sponge, Spongia (Heterofibria) sp. Org. Biomol. Chem. 2010, 8, 3188–3194. [Google Scholar] [CrossRef]
- Dai, J.; Sorribas, A.; Yoshida, W.Y.; Kelly, M.; Williams, P.G. Xestosaprols from the Indonesian marine sponge Xestospongia sp. J. Nat. Prod. 2010, 73, 1188–1191. [Google Scholar] [CrossRef]
- Plaza, A.; Keffer, J.L.; Lloyd, J.R.; Colin, P.L.; Bewley, C.A. Paltolides A–C, Anabaenopeptin-type peptides from the Palau sponge Theonella swinhoei. J. Nat. Prod. 2010, 73, 485–488. [Google Scholar] [CrossRef]
- Wei, X.; Nieves, K.; Rodríguez, A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the Caribbean sea sponge Neopetrosia proxima. Bioorg. Med. Chem. Lett. 2010, 20, 5905–5908. [Google Scholar] [CrossRef]
- Fan, G.; Li, Z.; Shen, S.; Zeng, Y.; Yang, Y.; Xu, M.; Bruhn, T.; Bruhn, H.; Morschhäuser, J.; Bringmann, G.; Lin, W. Baculiferins A–O, O-sulfated pyrrole alkaloids with anti-HIV-1 activity, from the Chinese marine sponge Iotrochota baculifera. Bioorg. Med. Chem. 2010, 18, 5466–5474. [Google Scholar]
- Schmitt, S.; Hentschel, U.; Taylor, M.W. Deep sequencing reveals diversity and community structure of complex microbiota in five Mediterranean sponges. Hydrobiologia 2012, 687, 341–351. [Google Scholar] [CrossRef]
- Erwin, P.M.; López-Legentil, S.; González-Pech, R.; Turon, X. A specific mix of generalists: Bacterial symbionts in Mediterranean Ircinia spp. FEMS Microbiol. Ecol. 2012, 79, 619–637. [Google Scholar] [CrossRef] [Green Version]
- Thiel, V.; Leininger, S.; Schmaljohann, R.; Brümmer, F.; Imhoff, J.F. Sponge-specific bacterial associations of the Mediterranean sponge Chondrilla nucula (Demospongiae, Tetractinomorpha). Microb. Ecol. 2007, 54, 101–111. [Google Scholar] [CrossRef]
- Perdicaris, S.; Vlachogianni, T.; Valavanidis, A. Bioactive natural substances from marine sponges: New developments and prospects for future pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 1–8. [Google Scholar]
- Sabdono, A. Microbial symbionts in marine sponges: Marine natural product factory. J. Coast. Dev. 2011, 11, 57–61. [Google Scholar]
- Laird, S.; Monagle, C.; Johnston, S. Queensland biodiscovery collaboration: The Griffith University AstraZeneca Partnership for natural product discovery: An access and benefit sharing case study. In An Access and Benefit Sharing Case Study; United Nations University, Institute of Advanced Studies: Yokohama, Japan, 2008. [Google Scholar]
- Mittermeier, R.A.; Goettsch Mittermeier, C. Megadiversity: Earth’s Biologically Wealthiest Nations. Megadiversity: Most Biological Rich Countries of the World; Cemex: San Pedro Garza García, Mexico, 1997. [Google Scholar]
- Tolley, M. UNEP-WCMC World Conservation Monitoring Centre Website; UNEP: Cambridge, UK, 2011. [Google Scholar]
- Butler, A.J.; Rees, T.; Beesley, P.; Bax, N.J. Marine biodiversity in the Australian region. PLoS One 2010, 5, e11831. [Google Scholar]
- Hughes, T.P.; Bellwood, D.R.; Connolly, S.R. Biodiversity hotspots, centres of endemicity, and the conservation of coral reefs. Ecol. Lett. 2002, 5, 775–784. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef]
- Wernberg, T.; Russell, B.D.; Moore, P.J.; Ling, S.D.; Smale, D.A.; Campbell, A.; Coleman, M.A.; Steinberg, P.D.; Kendrick, G.A.; Connell, S.D. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 2011, 400, 7–16. [Google Scholar] [CrossRef]
- Ward, T.M.; Sorokin, S.J.; Currie, D.R.; Rogers, P.J.; McLeay, L.J. Epifaunal assemblages of the eastern Great Australian Bight: Effectiveness of a benthic protection zone in representing regional biodiversity. Cont. Shelf Res. 2006, 26, 25–40. [Google Scholar] [CrossRef]
- Fromont, J.; Vanderklift, M.A.; Kendrick, G.A. Marine Sponges of the Dampier Archipelago, Western Australia: Patterns of Species Distributions, Abundance and Diversity; Springer: Berlin/Heidelberg, Germany, 2006; Volume 15, pp. 3731–3750. [Google Scholar]
- Hooper, J.; Kennedy, J.A. Small-scale patterns of sponge biodiversity (Porifera) from the Sunshine Coast reefs, eastern Australia. Invertebr. Syst. 2002, 16, 637–653. [Google Scholar] [CrossRef]
- Hooper, J.N.; Kennedy, J.A.; Quinn, R.J. Biodiversity “hotspots”, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera). Biodivers. Conserv. 2002, 11, 851–885. [Google Scholar] [CrossRef]
- Heyward, A.A.; Fromont, J.J.; Schoenberg, C.C.; Colquhoun, J.J.; Radford, B.B.; Gomez, O.O. The sponge gardens of Ningaloo Reef, Western Australia. Open Mar. Biol. 2010, 4, 3–11. [Google Scholar]
- Fromont, J.; Althaus, F.; McEnnulty, F.R.; Williams, A.; Salotti, M.; Gomez, O.; Gowlett-Holmes, K. Living on the edge: The sponge fauna of Australia’s southwestern and northwestern deep continental margin. Hydrobiologia 2012, 687, 127–142. [Google Scholar] [CrossRef]
- Williams, A.; Althaus, F.; Dunstan, P.K.; Poore, G.C.; Bax, N.J.; Kloser, R.J.; McEnnulty, F.R. Scales of habitat heterogeneity and megabenthos biodiversity on an extensive Australian continental margin (100–1100 m depths). Mar. Ecol. 2010, 31, 222–236. [Google Scholar] [CrossRef]
- Evans-Illidge, E.A.; Logan, M.; Doyle, J.; Fromont, J.; Battershill, C.N.; Ericson, G.; Wolff, C.W.; Muirhead, A.; Kearns, P.; Abdo, D. Phylogeny drives large scale patterns in australian marine bioactivity and provides a new chemical ecology rationale for future biodiscovery. PLoS One 2013, 8, e73800. [Google Scholar]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; Bell, J.J.; Davy, S.K.; Hoggard, M.; Taylor, M.W. Influence of environmental variation on symbiotic bacterial communities of two temperate sponges. FEMS Microbiol. Ecol. 2014, 88, 516–527. [Google Scholar] [CrossRef]
- Schmitt, S.; Tsai, P.; Bell, J.; Fromont, J.; Ilan, M.; Lindquist, N.; Perez, T.; Rodrigo, A.; Schupp, P.J.; Vacelet, J. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. Int. Soc. Micob. Ecol. J. 2012, 6, 564–576. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539-4577. https://doi.org/10.3390/md12084539
Mehbub MF, Lei J, Franco C, Zhang W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Marine Drugs. 2014; 12(8):4539-4577. https://doi.org/10.3390/md12084539
Chicago/Turabian StyleMehbub, Mohammad Ferdous, Jie Lei, Christopher Franco, and Wei Zhang. 2014. "Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives" Marine Drugs 12, no. 8: 4539-4577. https://doi.org/10.3390/md12084539
APA StyleMehbub, M. F., Lei, J., Franco, C., & Zhang, W. (2014). Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Marine Drugs, 12(8), 4539-4577. https://doi.org/10.3390/md12084539