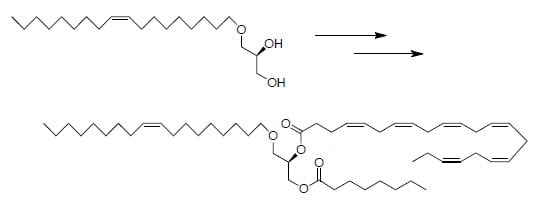
3.1. General
1H and
13C NMR spectra were recorded on a Bruker Avance 400 spectrometer using CDCl
3 as a solvent. Chemical shifts (δ) are reported in parts per million (ppm) and the coupling constants (
J) in Hertz (Hz). The following abbreviations are used to decribe the multiplicity: s, singlet; d, doublet; t, triplet; dd, doublet of doublets; m, multiplet. In the assignment parts of the
1H and
13C NMR spectra, SFA refers to the saturated fatty acyl group. The number of carbon nuclei behind each
13C signal is indicated in parentheses after each chemical shift value, when there is more than one carbon responsible for the peak. For all
13C NMR peaks, one digit after decimal point is provided except for the carbonyl carbons where two digits after the decimal point are provided to support data expressed in
Table 4. All infrared (IR) spectra were conducted on a Nicolet Avatar 360 FT-IR (E.S.P.) Spectrophotometer using neat liquid on a ZnSe plate. The optical activities were measured on an Autopol V from Rudolph Research Analytical, Hackettstown, NJ, USA. Melting points were determined on a Büchi 520 melting point apparatus and are uncorrected. The high-resolution mass spectra (HRMS) were acquired on a Bruker micrOTOF-Q mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) equipped with an E-spray atmospheric pressure ionization chamber (ESI) ((Bruker Daltonik GmbH, Bremen, Germany). All data analysis was done on a Bruker software ((Bruker Daltonik GmbH, Bremen, Germany).
All chemicals and solvents were used without further purification unless otherwise stated. EDAC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) was obtained from Sigma-Aldrich (Steinheim, Germany). Hexanoic acid (98%, zur synthese) and decanoic acid (98%, zur synthese) were obtained from Merck (Darmstadt, Germany) and hexadecanoic acid (99%) from Fluka (Buchs, Switzerland). Octanoic acid (>99.5%), dodecanoic acid (>99.5%), tetradecanoic acid (>99.5%) and 4-dimethylaminopyridine (DMAP, 99%) were obtained from Acros Organics (Geel, Belgium). Dichloromethane and benzene were obtained HPLC grade from Sigma-Aldrich (Steinheim, Germany). Column chromatography was performed on Silica gel 60 (Silicycle, Ontario, CA, USA). Reactions were monitered by TLC on Silica gel 60 F254 (Silicycle, Ontario, CA, USA), with detection by quenching of fluorescence, rhodamine 6G in CH3OH and/or with phosphomolybdic acid in ethanol.
3.1.1. Synthesis of 1-O-Hexadecyl-2-hexanoyl-3-eicosapentaenoyl-sn-glycerol (4a)
To a solution of (
R)-1-
O-hexadecyl-3-eicosapentaenoyl-
sn-glycerol
4 (67 mg, 0.112 mmol) and hexanoic acid (15 mg, 0.129 mmol) in CH
2Cl
2 (1 mL) were added DMAP (11 mg, 0.090 mmol) and EDAC (32 mg, 0.167 mmol). The resulting solution was stirred at r.t. for 12 h and the solvent then removed under reduced pressure. The residue was then purified by short silica column chromatography (CH
2Cl
2) to afford product
4a (74 mg, 0.106 mmol) as pale yellow oil, yield 95%.
−8.4 (
c 0.91, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.28 (m, 10H, =C
H), 5.22–5.17 (m, 1H, CH
2C
HCH
2), 4.34 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.17 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.6 Hz,
J = 5.3 Hz, CHC
H2O), 3.47–3.38 (2xdt, 2H,
J = 9.3 Hz,
J = 6.6 Hz, OC
H2CH
2), 2.88–2.76 (m, 8H, =CC
H2C=), 2.32 (2xt, 4H,
J = 7.6 Hz,
J = 7.5 Hz, C
H2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CC
H2CH
3 and =CC
H2CH
2), 1.70 (quintet (br), 2H,
J = 7.5 Hz, C
H2CH
2COO in EPA), 1.66–1.59 (m, 2H, C
H2CH
2COO in SFA), 1.57–1.50 (quintet (br), 2H,
J = 7.0 Hz, OCH
2C
H2), 1.35–1.20 (m, 30H, C
H2), 0.97 (t, 3H,
J = 7.5 Hz, C
H3 in EPA), 0.89 (t, 3H,
J = 6.9 Hz, C
H3 in SFA), 0.88 (t, 3H,
J = 7.0 Hz, C
H3 in ether) ppm (
Supplementary Information, Figures S2 and S4–S7);
13C NMR (CDCl
3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.1, 68.9, 62.9, 34.3, 33.5, 31.9, 31.2, 29.7 (7), 29.6 (3), 29.5, 29.4, 26.5, 26.0, 25.6 (2), 25.5, 24.7, 24.6, 22.7, 22.3, 20.6, 14.3, 14.1, 13.9 ppm (
Supplementary Information, Figure S3); IR (ZnSe) 3013 (s, CH), 2924 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
45H
78O
5 (M + H
+) 699.5922, found 699.5909.
3.1.2. Synthesis of 1-O-Hexadecyl-2-octanoyl-3-eicosapentaenoyl-sn-glycerol (4b)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-eicosapentaenoyl-sn-glycerol 4 (114 mg, 0.190 mmol), octanoic acid (37 mg, 0.257 mmol), DMAP (25 mg, 0.205 mmol) and EDAC (51 mg, 0.266 mmol) in 2 mL CH2Cl2. The product 4b (126 mg, 0.173 mmol) was afforded as pale yellow oil, yield 91%. −8.6 (c 0.90, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.76 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.66–1.58 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (quintet (br), 2H, J = 7.0 Hz, OCH2CH2), 1.35–1.20 (m, 34H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.9 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.1, 68.9, 62.9, 34.3, 33.5, 31.9, 31.7, 29.7 (7), 29.6 (3), 29.5, 29.4, 29.0, 28.9, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7, 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1740 (vs, C=O) cm−1; HRMS m/z calcd. for C47H82O5 (M + NH4+) 744.6501, found 744.6466.
3.1.3. Synthesis of 1-O-Hexadecyl-2-decanoyl-3-eicosapentaenoyl-sn-glycerol (4c)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-eicosapentaenoyl-sn-glycerol 4 (126 mg, 0.210 mmol), decanoic acid (35 mg, 0.203 mmol), DMAP (30 mg, 0.245 mmol) and EDAC (68 mg, 0.355 mmol) in 1 mL CH2Cl2. The product 4c (142 mg, 0.188 mmol) was afforded as colorless oil, yield 90%. −8.1 (c 0.99, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (quinted (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.20 (m, 38H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (7), 29.6 (3), 29.5, 29.4 (2), 29.3 (2), 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C49H86O5 (M + NH4+) 772.6814, found 772.6781.
3.1.4. Synthesis of 1-O-Hexadecyl-2-dodecanoyl-3-eicosapentaenoyl-sn-glycerol (4d)
The same procedure was followed as for 4a except using (R)-1-O-hexadecyl-3-eicosapentaenoyl-sn-glycerol 4 (60 mg, 0.100 mmol), dodecanoic acid (24 mg, 0.120 mmol), DMAP (9 mg, 0.070 mmol) and EDAC (28 mg, 0.146 mmol) in 1 mL CH2Cl2. The product 4d (77 mg, 0.098 mmol) was afforded as colorless oil, yield 98%. −7.6 (c 0.85, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quinted (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.20 (m, 42H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (8), 29.6 (4), 29.5 (2), 29.4, 29.3 (2), 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C51H90O5 (M + H+) 783.6861, found 783.6857.
3.1.5. Synthesis of 1-O-Hexadecyl-2-tetradecanoyl-3-eicosapentaenoyl-sn-glycerol (4e)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-eicosapentaenoyl-sn-glycerol 4 (50 mg, 0.083 mmol), tetradecanoic acid (23 mg, 0.101 mmol), DMAP (7 mg, 0.057 mmol) and EDAC (23 mg, 0.112 mmol) in 1 mL CH2Cl2. The product 4e (59 mg, 0.073 mmol) was afforded as colorless oil, yield 88%. −8.3 (c 0.80, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quinted (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.20 (m, 46H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (9), 29.6 (5), 29.5 (2), 29.4 (2), 29.3, 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR(ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C53H94O5 (M + H+) 828.7440, found 828.7439.
3.1.6. Synthesis of 1-O-Hexadecyl-2-hexadecanoyl-3-eicosapentaenoyl-sn-glycerol (4f)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-eicosapentaenoyl-sn-glycerol 4 (56 mg, 0.093 mmol), hexadecanoic acid (28 mg, 0.109 mmol), DMAP (14 mg, 0.115 mmol) and EDAC (26 mg, 0.136 mmol) in 1 mL CH2Cl2. The product 4f (76 mg, 0.091 mmol) was afforded as colorless oil, yield 97%. −7.5 (c 1.2, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.76 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quinted (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.20 (m, 50H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (11), 29.6 (5), 29.5 (2), 29.4 (2), 29.3, 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1: HRMS m/z calcd. for C55H98O5 (M + NH4+) 856.7753, found 856.7751.
3.1.7. Synthesis of 1-O-Hexadecyl-2-hexanoyl-3-docosahexaenoyl-sn-glycerol (5a)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-docosahexaenoyl-sn-glycerol 5 (32 mg, 0.053 mmol), hexanoic acid (8 mg, 0.069 mmol), DMAP (8 mg, 0.069 mmol) and EDAC (16 mg, 0.083 mmol) in 1 mL CH2Cl2. The product 5a (34 mg, 0.047 mmol) was afforded as colorless oil, yield 88%. −8.8 (c 0.95, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.18 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.4 Hz, CHCH2O), 3.48–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.7 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.36 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.66–1.57 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (m, 2H, OCH2CH2), 1.35–1.21 (m, 30H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.89 (t, 3H, J = 6.8 Hz, CH3 in SFA), 0.88 (t, 3H, J = 7.0 Hz, CH3 in ether); 13C NMR (CDCl3) δ 173.17 (β, C=O in SFA), 172.72 (α, C=O in DHA), 132.1, 129.4, 128.6, 128.3 (3), 128.2, 128.1 (2), 127.9, 127.8, 127.0, 71.8, 70.1, 69.0, 63.0, 34.3, 34.0, 32.0, 31.3, 29.7 (9), 29.6, 29.5, 29.4, 26.1, 25.7 (2), 25.6 (2), 24.7, 22.7 (2), 22.4, 20.6, 14.3, 14.2, 14.0 ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C47H80O5 (M + H+) 725.6079, found 725.6085.
3.1.8. Synthesis of 1-O-Hexadecyl-2-octanoyl-3-docosahexaenoyl-sn-glycerol (5b)
The same procedure was followed as desribed for
4a using (
R)-1-
O-hexadecyl-3-docosahexaenoyl-
sn-glycerol
5 (65 mg, 0.103 mmol), octanoic acid (21 mg, 0.146 mmol), DMAP (9 mg, 0.072 mmol) and EDAC (30 mg, 0.156 mmol) in 1 mL CH
2Cl
2. The product
5b (73 mg, 0.097 mmol) was afforded as colorless oil, yield 94%.
−8.6 (
c 1.0, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.28 (m, 12H, =C
H), 5.22–5.17 (m, 1H, CH
2C
HCH
2), 4.35 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.18 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.6 Hz,
J 5.4 Hz, CHC
H2O), 3.49–3.37 (2xdt, 2H,
J = 9.3,
J = 6.7 Hz, OC
H2CH
2), 2.88–2.80 (m, 10H, =CC
H2C=), 2.41–2.35 (m, 4H, C
H2C
H2COO in DHA), 2.32 (t, 2H,
J = 7.5 Hz, C
H2COO in SFA), 2.11–2.04 (m, 2H, =CC
H2CH
3), 1.65-1.58 (m, 2H, C
H2CH
2COO in SFA), 1.58–1.50 (m, 2H, OCH
2C
H2), 1.35–1.22 (m, 34H, C
H2), 0.97 (t, 3H,
J = 7.5 Hz, C
H3 in DHA), 0.88 (t, 6H,
J = 6.8 Hz, C
H3) ppm (
Supplementary Information, Figures S8 and S10–S13);
13C NMR (CDCl
3) δ 173.18 (β, C=O in SFA), 172.74 (α, C=O in DHA), 132.1, 129.4, 128.6, 128.3 (3), 128.1 (3), 127.9, 127.8, 127.1, 71.8, 70.1, 69.0, 63.0, 34.4, 34.0, 32.0, 31.7, 29.7 (8), 29.6, 29.5, 29.4, 29.1, 29.0, 26.1, 25.7 (3), 25.6 (2), 25.0, 22.7 (3), 20.6, 14.3, 14.2, 14.1 ppm (
Supplementary Information, Figure S9); IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
49H
84O
5 (M + H
+) 753.6392, found 753.6374.
3.1.9. Synthesis of 1-O-Hexadecyl-2-decanoyl-3-docosahexaenoyl-sn-glycerol (5c)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-docosahexaenoyl-sn-glycerol 5 (156 mg, 0.249 mmol), decanoic acid (64 mg, 0.371 mmol), DMAP (35 mg, 0.286 mmol) and EDAC (71 mg, 0.370 mmol) in 1 mL CH2Cl2. The product 5c (166 mg, 0.212 mmol) was afforded as colorless oil, yield 85%. −8.2 (c 0.97, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.27 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 38H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.13 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.3, 128.5, 128.3, 128.2 (2), 128.1, 128.0 (2), 127.8 (2), 127.0, 71.7, 70.0, 68.9, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (7), 29.6 (2), 29.5 (2), 29.4 (2), 29.3 (2), 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 22.6, 20.5, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1. HRMS m/z calcd. for C51H88O5 (M + NH4+) 798.6970, found 798.6958.
3.1.10. Synthesis of 1-O-Hexadecyl-2-dodecanoyl-3-docosahexaenoyl-sn-glycerol (5d)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-docosahexaenoyl-sn-glycerol 5 (60 mg, 0.095 mmol), dodecanoic acid (23 mg, 0.115 mmol), DMAP (15 mg, 0.123 mmol) and EDAC (27 mg, 0.141 mmol) in 1 mL CH2Cl2. The product 5d (67 mg, 0.082 mmol) was afforded as colorless oil, yield 87%. −8.2 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.4 Hz, CHCH2O), 3.46–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.65–1.56 (m, 2H, CH2CH2COO in SFA), 1.56–1.50 (m, 2H, OCH2CH2), 1.35–1.21 (m, 42H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.13 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.4, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.1, 69.0, 63.0, 34.4, 34.0, 31.9 (2), 29.7 (7), 29.6 (5), 29.5 (2), 29.4, 29.3 (2), 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3014 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C53H92O5 (M + H+) 809.7018, found 809.7016.
3.1.11. Synthesis of 1-O-Hexadecyl-2-tetradecanoyl-3-docosahexaenoyl-sn-glycerol (5e)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-docosahexaenoyl-sn-glycerol 5 (148 mg, 0.236 mmol), tetradecanoic acid (60 mg, 0.263 mmol), DMAP (31 mg, 0.254 mmol) and EDAC (67 mg, 0.349 mmol) in 2 mL CH2Cl2. The product 5e (185 mg, 0.221 mmol) was afforded as colorless oil, yield 94%. −8.0 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J 5.3 Hz, CHCH2O), 3.46–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41-2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 46H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 172.70 (α, C=O in DHA), 132.0, 129.3, 128.5, 128.3, 128.2 (2), 128.1 (2), 128.0, 127.8 (2), 127.0, 71.7, 70.0, 68.9, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (11), 29.6 (2), 29.5 (3), 29.4 (2), 29.3, 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C55H96O5 (M + H+) 837.7331, found 837.7315.
3.1.12. Synthesis of 1-O-Hexadecyl-2-hexadecanoyl-3-docosahexaenoyl-sn-glycerol (5f)
The same procedure was followed as described for 4a using (R)-1-O-hexadecyl-3-docosahexaenoyl-sn-glycerol 5 (103 mg, 0.164 mmol), hexadecanoic acid (49 mg, 0.191 mmol), DMAP (21 mg, 0.172 mmol) and EDAC (47 mg, 0.245 mmol) in 1 mL CH2Cl2. The product 5f (139 mg, 0.161 mmol) was afforded as colorless oil, yield 96%. −8.4 (c 0.89, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.46–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.35–1.21 (m, 50H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.13 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.7, 70.0, 69.0, 63.0, 34.4, 34.0, 31.9 (2), 29.7 (12), 29.6 (4), 29.5 (2), 29.4 (2), 29.3, 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2921 (vs, CH), 2852 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C57H100O5 (M + NH4+) 882.7909, found 882.7888.
3.1.13. Synthesis of 1-O-Octadecyl-2-hexanoyl-3-eicosapentaenoyl-sn-glycerol (6a)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-eicosapentaenoyl-sn-glycerol 6 (115 mg, 0.183 mmol), hexanoic acid (21 mg, 0.181 mmol), DMAP (23 mg, 0.188 mmol) and EDAC (50 mg, 0.261 mmol) in 1 mL CH2Cl2. The product 6a (119 mg, 0.164 mmol) was afforded as colorless oil, yield 90%. −8.3 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9, J 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.79 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.70 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.66–1.59 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (quinted (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.25 (m, 34H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.89 (t, 3H, J = 6.9 Hz, CH3 in SFA), 0.88 (t, 3H, J = 7.0 Hz, CH3 in ether) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.1, 68.9, 62.9, 34.3, 33.5, 31.9, 31.2, 29.7 (8), 29.6 (3), 29.5, 29.4, 26.5, 26.0, 25.6 (3), 25.5, 24.7, 24.6, 22.7, 22.3, 20.6, 14.3, 14.1, 13.9 ppm; IR (ZnSe) 3013 (s, CH), 2921 (vs, CH), 2851 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C47H82O5 (M + NH4+) 744.6501, found 744.6481.
3.1.14. Synthesis of 1-O-Octadecyl-2-octanoyl-3-eicosapentaenoyl-sn-glycerol (6b)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-eicosapentaenoyl-sn-glycerol 6 (65 mg, 0.103 mmol), octanoic acid (20 mg, 0.139 mmol), DMAP (13 mg, 0.107 mmol) and EDAC (33 mg, 0.172 mmol) in 1 mL CH2Cl2. The product 6b (76 mg, 0.101 mmol) was afforded as pale yellow oil, yield 98%. −8.0 (c 0.83, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.5 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.66–1.58 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (quintet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.35–1.20 (m, 38H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.9 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9, 31.7, 29.7 (9), 29.6 (2), 29.5, 29.4, 29.0, 28.9, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.6, 24.7, 22.7, 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C49H86O5 (M + NH4+) 772.6800, found 772.6790.
3.1.15. Synthesis of 1-O-Octadecyl-2-decanoyl-3-eicosapentaenoyl-sn-glycerol (6c)
The same procedure was followed as described for
4a using (
R)-1-
O-octadecyl-3-eicosapentaenoyl-
sn-glycerol
6 (82 mg, 0.130 mmol), decanoic acid (26 mg, 0.151 mmol), DMAP (20 mg, 0.163 mmol) and EDAC (41 mg, 0.214 mmol) in 1 mL CH
2Cl
2. The product
6c (97 mg, 0.124 mmol) was afforded as pale yellow oil, yield 95%.
−7.9 (
c 0.82, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.28 (m, 10H, =C
H), 5.22–5.17 (m, 1H, CH
2C
HCH
2), 4.34 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.17 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.7 Hz,
J = 5.4 Hz, CHC
H2O), 3.47–3.38 (2xdt, 2H,
J = 9.3 Hz,
J = 6.7 Hz, OC
H2CH
2), 2.86–2.79 (m, 8H, =CC
H2C=), 2.32 (2xt, 4H,
J = 7.6, Hz
J = 7.5 Hz, C
H2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CC
H2CH
3 and =CC
H2CH
2), 1.69 (quintet (br), 2H,
J = 7.5 Hz, C
H2CH
2COO in EPA), 1.65–1.58 (m, 2H, C
H2CH
2COO in SFA), 1.58–1.50 (m, 2H, OCH
2C
H2), 1.38–1.21 (m, 42H, C
H2), 0.97 (t, 3H,
J = 7.5 Hz, C
H3 in EPA), 0.88 (t, 6H,
J = 6.8 Hz, C
H3) ppm (
Supplementary Information, Figure S14);
13C NMR (CDCl
3) δ 173.14 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.1, 69.0, 62.9, 34.4, 33.5, 31.9 (2), 29.7 (11), 29.6 (2), 29.5, 29.4, 29.3 (2), 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm (
Supplementary Information, Figure S15); IR (ZnSe) 3013 (s, CH), 2921 (vs, CH), 2852 (s, CH), 1741 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
51H
90O
5 (M + NH
4+) 800.7127, found 800.7131.
3.1.16. Synthesis of 1-O-Octadecyl-2-dodecanoyl-3-eicosapentaenoyl-sn-glycerol (6d)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-eicosapentaenoyl-sn-glycerol 6 (98 mg, 0.156 mmol), dodecanoic acid (30 mg, 0.150 mmol), DMAP (20 mg, 0.164 mmol) and EDAC (49 mg, 0.256 mmol) in 1 mL CH2Cl2. The product 6d (115 mg, 0.142 mmol) was afforded as pale yellow oil, yield 94%. −7.5 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.7 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 46H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.20 (β, C=O in SFA), 173.18 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 68.9, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (9), 29.6 (5), 29.5 (2), 29.4, 29.3 (2), 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C53H94O5 (M + NH4+) 828.7440, found 828.7437.
3.1.17. Synthesis of 1-O-Octadecyl-2-tetradecanoyl-3-eicosapentaenoyl-sn-glycerol (6e)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-eicosapentaenoyl-sn-glycerol 6 (58 mg, 0.093 mmol), tetradecanoic acid (24 mg, 0.105 mmol), DMAP (9 mg, 0.073 mmol) and EDAC (26 mg, 0.136 mmol) in 1 mL CH2Cl2. The product 6e (68 mg, 0.081 mmol) was afforded as pale yellow oil, yield 87%. −7.4 (c 0.95, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.79 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 50H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9 (2), 29.7 (14), 29.6 (2), 29.5, 29.4 (2), 29.3 (2), 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C55H98O5 (M + H+) 839.7487, found 839.7486.
3.1.18. Synthesis of 1-O-Octadecyl-2-hexadecanoyl-3-eicosapentaenoyl-sn-glycerol (6f)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-eicosapentaenoyl-sn-glycerol 6 (73 mg, 0.116 mmol), hexadecanoic acid (31 mg, 0.121 mmol), DMAP (18 mg, 0.147 mmol) and EDAC (39 mg, 0.203 mmol) in 1 mL CH2Cl2. The product 6f (90 mg, 0.104 mmol) was afforded as colorless oil, yield 90%. −6.6 (c 0.93, benzene). 1H NMR (400 MHz, CDCl3) δ 5.44–5.28 (m, 10H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.89–2.79 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.57 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 54H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.16 (β, C=O in SFA), 173.13 (α, C=O in EPA), 132.0, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.8, 70.1, 69.0, 62.9, 34.4, 33.5, 31.9 (2), 29.7 (17), 29.6, 29.5 (2), 29.4 (2), 29.3, 29.1, 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2922 (vs, CH), 2852 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C57H102O5 (M + H+) 867.7800, found 867.7774.
3.1.19. Synthesis of1-O-Octadecyl-2-hexanoyl-3-docosahexaenoyl-sn-glycerol (7a)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-docosahexaenoyl-sn-glycerol 7 (68 mg, 0.104 mmol), hexanoic acid (17 mg, 0.146 mmol), DMAP (10 mg, 0.082 mmol) and EDAC (29 mg, 0.151 mmol) in 1 mL CH2Cl2. The product 7a (71 mg, 0.098 mmol) was afforded as colorless oil, yield 94%. −8.5 (c 0.95, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.18 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.4 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.7 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.66–1.59 (m, 2H, CH2CH2COO in SFA), 1.59–1.50 (m, 2H, OCH2CH2), 1.35–1.21 (m, 34H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.89 (t, 3H, J = 6.8 Hz, CH3 in SFA), 0.88 (t, 3H, J = 7.0 Hz, CH3 in ether) ppm; 13C NMR (CDCl3) δ 173.10 (β, C=O in SFA), 172.66 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.1, 69.0, 63.0, 34.3, 34.0, 31.9, 31.2, 29.7 (9), 29.6 (3), 29.5, 29.4, 26.0, 25.6 (3), 25.5, 24.6, 22.7 (2), 22.3, 20.6, 14.3, 14.1, 13.9 ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C49H84O5 (M + H+) 753.6392, found 753.6379.
3.1.20. Synthesis of 1-O-Octadecyl-2-octanoyl-3-docosahexaenoyl-sn-glycerol (7b)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-docosahexaenoyl-sn-glycerol 7 (66 mg, 0.100 mmol), octanoic acid (18 mg, 0.125 mmol), DMAP (17 mg, 0.139 mmol) and EDAC (30 mg, 0.156 mmol) in 1 mL CH2Cl2. The product 7b (73 mg, 0.093 mmol) was afforded as pale yellow oil, yield 94%. −8.1 (c 0.94, benzene). 1H NMR (400 MHz, CDCl3 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.7 Hz, J = 5.5 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.7 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.37–1.20 (m, 38H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.11 (β, C=O in SFA), 172.66 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9, 31.7, 29.7 (9), 29.6 (2), 29.5, 29.4, 29.0, 28.9, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C51H88O5 (M + NH4+) 798.6970, found 798.6964.
3.1.21. Synthesis of 1-O-Octadecyl-2-decanoyl-3-docosahexaenoyl-sn-glycerol (7c)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-docosahexaenoyl-sn-glycerol 7 (157 mg, 0.240 mmol), decanoic acid (50 mg, 0.290 mmol), DMAP (31 mg, 0.254 mmol) and EDAC (77 mg, 0.402 mmol) in 1 mL CH2Cl2. The product 7c (176 mg, 0.218 mmol) was afforded as colorless oil, yield 91%. −7.9 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.4 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.7 Hz, OCH2CH2), 2.91–2.80 (m, 10H, =CCH2C=), 2.42–2.37 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, J = 7.4 Hz, =CCH2CH3), 1.65–-1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.39–1.21 (m, 42H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.11 (β, C=O in SFA), 172.66 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (11), 29.6 (2), 29.5, 29.4 (2), 29.3 (2), 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3014 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C53H92O5 (M + H+) 809.7018, found 809.7011.
3.1.22. Synthesis of 1-O-Octadecyl-2-dodecanoyl-3-docosahexaenoyl-sn-glycerol (7d)
The same procedure was followed as described for
4a using (
R)-1-
O-octadecyl-3-docosahexaenoyl-
sn-glycerol
7 (100 mg, 0.153 mmol), dodecanoic acid (33 mg, 0.165 mmol), DMAP (22 mg, 0.180 mmol) and EDAC (50 mg, 0.261 mmol) in 1 mL CH
2Cl
2. The product
7d (112 mg, 0.134 mmol) was afforded as colorless oil, yield 88%.
−7.7 (
c 1.0, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.28 (m, 12H, =C
H), 5.22-5.17 (m, 1H, CH
2C
HCH
2), 4.35 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.17 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.6 Hz,
J = 5.3 Hz, CHC
H2O), 3.47–3.37 (2xdt, 2H,
J = 9.3 Hz,
J = 6.6 Hz, OC
H2CH
2), 2.88–2.80 (m, 10H, =CC
H2C=), 2.41–2.35 (m, 4H, C
H2C
H2COO in DHA), 2.32 (t, 2H,
J = 7.5 Hz, C
H2COO in SFA), 2.11–2.04 (quintet, 2H,
J = 7.5 Hz, =CC
H2CH
3), 1.65–1.57 (m, 2H, C
H2CH
2COO in SFA), 1.57–1.50 (m, 2H, OCH
2C
H2), 1.35–1.20 (m, 46H, C
H2), 0.97 (t, 3H,
J =.5 Hz, C
H3 in DHA), 0.88 (t, 6H,
J = 6.8 Hz, C
H3) ppm (
Supplementary Information, Figure S16);
13C NMR (CDCl
3) δ 173.12 (β, C=O in SFA), 172.67 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 68.9, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (10), 29.6 (5), 29.5 (2), 29.4, 29.3 (2), 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm (
Supplementary Information, Figure S17); IR (ZnSe) 3014 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
55H
96O
5 (M + NH
4+) 854.7596, found 854.7607.
3.1.23. Synthesis of 1-O-Octadecyl-2-tetradecanoyl-3-docosahexaenoyl-sn-glycerol (7e)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-docosahexaenoyl-sn-glycerol 7 (59 mg, 0.092 mmol), tetradecanoic acid (23 mg, 0.101 mmol), DMAP (10 mg, 0.082 mmol) and EDAC (26 mg, 0.136 mmol) in 1 mL CH2Cl2. The product 7e (73 mg, 0.084 mmol) was afforded as pale yellow oil, yield 94%. −7.3 (c 0.91, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (quintet, 2H, J = 7.5 Hz, =CH2CH3), 1.65–1.57 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 50H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.12 (β, C=O in SFA), 172.67 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 68.9, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (15), 29.6 (2), 29.5 (2), 29.4 (2), 29.3, 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3014 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1743 (vs, C=O) cm−1; HRMS m/z calcd. for C57H100O5 (M + H+) 865.7644, found 865.7625.
3.1.24. Synthesis of 1-O-Octadecyl-2-hexadecanoyl-3-docosahexaenoyl-sn-glycerol (7f)
The same procedure was followed as described for 4a using (R)-1-O-octadecyl-3-docosahexaenoyl-sn-glycerol 7 (57 mg, 0.087 mmol), hexadecanoic acid (25 mg, 0.097 mmol), DMAP (12 mg, 0.098 mmol) and EDAC (26 mg, 0.136 mmol) in 1 mL CH2Cl2. The product 7f (72 mg, 0.081 mmol) was afforded as pale yellow solid, yield 93%. Mp 29–31 °C. −7.2 (c 0.79, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (quintet, 2H, J = 7.5 Hz, =CCH2CH3), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.35–1.20 (m, 54H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.12 (β, C=O in SFA), 172.67 (α, C=O in DHA), 132.0, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 68.9, 63.0, 34.3, 34.0, 31.9 (2), 29.7 (17), 29.6, 29.5 (2), 29.4 (2), 29.3, 29.1, 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3014 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1743 (vs, C=O) cm−1; HRMS m/z calcd. for C59H104O5 (M + NH4+) 910.8222, found 910.8220.
3.1.25. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-hexanoyl-3-eicosapentaenoyl-sn-glycerol (8a)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-eicosapentaenoyl-sn-glycerol 8 (98 mg, 0.156 mmol), hexanoic acid (20 mg, 0.172 mmol), DMAP (18 mg, 0.147 mmol) and EDAC (50 mg, 0.261 mmol) in 1 mL CH2Cl2. The product 8a (102 mg, 0.141 mmol) was afforded as pale yellow oil, yield 90%. −7.7 (c 1.1, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6, Hz J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.79 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.06 (m, 4H, =CCH2CH3 and =CCH2CH2), 2.04–1.99 (m, 4H, =CCH2 in selachyl), 1.70 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.66–1.59 (m, 2H, CH2CH2COO in SFA), 1.59–1.50 (qunitet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.37–1.22 (m, 26H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.89 (t, 3H, J = 6.9 Hz, CH3 in SFA), 0.88 (t, 3H, J = 7.0 Hz, CH3 in ether) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.11 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9, 31.2, 29.8 (2), 29.7, 29.6, 29.5, 29.4, 29.3 (3), 27.2 (2), 26.5, 26.0, 25.6 (3), 25.5, 24.7, 24.6, 22.7, 22.3, 20.6, 14.3, 14.1, 13.9 ppm; IR (ZnSe) 3012 (s, CH), 2923 (vs, CH), 2854 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C47H80O5 (M + NH4+) 742.6344, found 742.6336.
3.1.26. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-octanoyl-3-eicosapentaenoyl-sn-glycerol (8b)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-eicosapentaenoyl-sn-glycerol 8 (60 mg, 0.096 mmol), octanoic acid (15 mg, 0.104 mmol), DMAP (11 mg, 0.090 mmol) and EDAC (26 mg, 0.136 mmol) in 1 mL CH2Cl2. The product 8b (62 mg, 0.082 mmol) was afforded as pale yellow oil, yield 86%. −7.5 (c 0.88, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 2.04–1.99 (m, 4H, =CCH2 in selachyl), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.63–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quintet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.39–1.22 (m, 30H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.9 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.11 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9, 31.7, 29.8 (2), 29.7, 29.6, 29.5 (2), 29.4, 29.3 (3), 29.0, 28.9, 27.2 (2), 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7, 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2924 (vs, CH), 2854 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C49H84O5 (M + NH4+) 770.6657, found 770.6657.
3.1.27. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-decanoyl-3-eicosapentaenoyl-sn-glycerol (8c)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-eicosapentaenoyl-sn-glycerol 8 (60 mg, 0.096 mmol), decanoic acid (18 mg, 0.104 mmol), DMAP (8 mg, 0.067 mmol) and EDAC (27 mg, 0.141 mmol) in 1 mL CH2Cl2. The product 8c (64 mg, 0.082 mmol) was afforded as pale yellow oil, yield 86%. −6.8 (c 0.95, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 2.04–1.99 (m, 4H, =CCH2 in selachyl), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quintet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.39–1.22 (m, 34H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.7 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9 (2), 29.8 (2), 29.7, 29.6, 29.5 (2), 29.4 (2), 29.3 (5), 29.1, 27.2 (2), 26.5, 26.0, 25.6 (2), 25.5, 24.9, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2923 (vs, CH), 2854 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C51H88O5 (M + NH4+) 798.6970, found 798.6940.
3.1.28. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-dodecanoyl-3-eicosapentaenoyl-sn-glycerol (8d)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-eicosapentaenoyl-sn-glycerol 8 (60 mg, 0.096 mmol), dodecanoic acid (21 mg, 0.105 mmol), DMAP (13 mg, 0.107 mmol) and EDAC (27 mg, 0.141 mmol) in 1 mL CH2Cl2. The product 8d (74 mg, 0.091 mmol) was afforded as pale yellow oil, yield 96%. −6.8 (c 0.87, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.38 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.87–2.77 (m, 8H, =CCH2C=), 2.32 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 2.04–1.99 (m, 4H, =CCH2 in selachyl), 1.69 (quinted (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quintet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.39–1.22 (m, 38H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.7 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9 (2), 29.8 (2), 29.7, 29.6 (3), 29.5 (3), 29.4, 29.3 (5), 29.1, 27.2 (2), 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C53H92O5 (M + H+) 809.7018, found 809.7008.
3.1.29. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-tetradecanoyl-3-eicosapentaenoyl-sn-glycerol (8e)
The same procedure was followed as described for
4a using (
R)-1-
O-(
Z)-octadec-9-enyl-3-eicosapentaenoyl-
sn-glycerol
8 (60 mg, 0.096 mmol), tetradecanoic acid (23 mg, 0.101 mmol), DMAP (10 mg, 0.082 mmol) and EDAC (29 mg, 0.150 mmol) in 1 mL CH
2Cl
2. The product
8e (72 mg, 0.086 mmol) was afforded as pale yellow oil, yield 90%.
−6.7 (
c 0.90, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.29 (m, 12H, =C
H), 5.22–5.17 (m, 1H, CH
2C
HCH
2), 4.34 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.17 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.6 Hz,
J = 5.3 Hz, CHC
H2O), 3.47–3.38 (2xdt, 2H,
J = 9.3 Hz,
J = 6.6 Hz, OC
H2CH
2), 2.88–2.77 (m, 8H, =CC
H2C=), 2.31 (2xt, 4H,
J = 7.6 Hz,
J = 7.5 Hz, C
H2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CC
H2CH
3 and =CC
H2CH
2), 2.04–1.99 (m, 4H, =CC
H2 in selachyl), 1.69 (quintet (br), 2H,
J = 7.5 Hz, C
H2CH
2COO in EPA), 1.65–1.58 (m, 2H, C
H2CH
2COO in SFA), 1.58–1.50 (quintet (br), 2H,
J = 6.8 Hz, OCH
2C
H2), 1.39–1.22 (m, 42H, C
H2), 0.97 (t, 3H,
J = 7.5 Hz, C
H3 in EPA), 0.88 (t, 6H,
J = 6.8 Hz, C
H3) ppm (
Supplementary Information, Figure S18);
13C NMR (CDCl
3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9 (2), 29.8 (2), 29.7 (3), 29.6 (2), 29.5 (3), 29.4 (2), 29.3 (5), 29.1, 27.2 (2), 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm (
Supplementary Information, Figure S19); IR (ZnSe) 3012 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
55H
96O
5 (M + NH
4+) 854.7596, found 854.7611.
3.1.30. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-hexadecanoyl-3-eicosapentaenoyl-sn-glycerol (8f)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-eicosapentaenoyl-sn-glycerol 8 (70 mg, 0.112 mmol), hexadecanoic acid (31 mg, 0.121 mmol), DMAP (13 mg, 0.107 mmol) and EDAC (33 mg, 0.172 mmol) in 1 mL CH2Cl2. The product 8f (83 mg, 0.095 mmol) was afforded as pale yellow oil, yield 86%. −6.7 (c 0.86, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 12H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.34 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.77 (m, 8H, =CCH2C=), 2.31 (2xt, 4H, J = 7.6 Hz, J = 7.5 Hz, CH2COO in EPA and SFA), 2.13–2.04 (m, 4H, =CCH2CH3 and =CCH2CH2), 2.04–1.99 (m, 4H, =CCH2 in selachyl), 1.69 (quintet (br), 2H, J = 7.5 Hz, CH2CH2COO in EPA), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (quintet (br), 2H, J = 6.8 Hz, OCH2CH2), 1.38–1.20 (m, 46H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in EPA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.15 (β, C=O in SFA), 173.12 (α, C=O in EPA), 132.0, 129.9, 129.8, 128.9 (2), 128.6, 128.3, 128.2 (2), 128.1, 127.9, 127.0, 71.7, 70.0, 69.0, 62.9, 34.3, 33.5, 31.9 (2), 29.8 (2), 29.7 (6), 29.6 (2), 29.5 (3), 29.4 (2), 29.3 (4), 29.1, 27.2 (2), 26.5, 26.0, 25.6 (2), 25.5, 25.0, 24.7, 22.7 (2), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3012 (s, CH), 2922 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C57H100O5 (M + NH4+) 882.7909, found 882.7901.
3.1.31. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-hexanoyl-3-docosahexaenoyl-sn-glycerol (9a)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-docosahexaenoyl-sn-glycerol 9 (99 mg, 0.152 mmol), hexanoic acid (20 mg, 0.172 mmol), DMAP (20 mg, 0.164 mmol) and EDAC (41 mg, 0.214 mmol) in 1 mL CH2Cl2. The product 9a (105 mg, 0.140 mmol) was afforded as colorless oil, yield 92%. −8.3 (c 0.83, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 14H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6, Hz J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.79 (m, 10H, =CCH2C=), 2.39–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.04 (m, 2H, =CCH2CH3), 2.03–1.98 (m, 4H, =CCH2 in selachyl), 1.66–1.60 (m, 2H, CH2CH2COO in SFA), 1.60–1.50 (m, 2H, OCH2CH2), 1.34–1.23 (m, 26H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.89 (t, 3H, J = 6.9 Hz, CH3 in SFA), 0.88 (t, 3H, J = 7.0 Hz, CH3 in ether) ppm; 13C NMR (CDCl3) δ 173.12 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9, 31.2, 30.9, 29.8 (2), 29.6, 29.5 (2), 29.4, 29.3 (3), 27.2 (2), 26.0, 25.6 (3), 25.5, 24.6, 24.6, 22.7, 22.3, 20.6, 14.3, 14.1, 13.9 ppm; IR (ZnSe) 3012 (s, CH), 2924 (vs, CH), 2854 (s, CH), 1741 (vs, C=O) cm−1; HRMS m/z calcd. for C49H82O5 (M + NH4+) 768.6501, found 768.6513.
3.1.32. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-octanoyl-3-docosahexaenoyl-sn-glycerol (9b)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-docosahexaenoyl-sn-glycerol 9 (92 mg, 0.141 mmol), octanoic acid (26 mg, 0.180 mmol), DMAP (17 mg, 0.139 mmol) and EDAC (39 mg, 0.203 mmol) in 1 mL CH2Cl2. The product 9b (98 mg, 0.126 mmol) was afforded as colorless oil, yield 89%. −7.3 (c 0.84, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 14H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.39–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.03 (m, 2H, =CCH2CH3), 2.03–1.98 (m, 4H, =CCH2 in selachyl), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.38–1.21 (m, 30H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.7 Hz, CH3 ppm; 13C NMR (CDCl3) δ 173.12 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9, 31.7, 30.9, 29.8 (2), 29.6, 29.5 (2), 29.4, 29.3 (3), 29.0, 28.9, 27.2 (2), 26.0, 25.6 (3), 25.5, 25.0, 22.7 (2), 22.6, 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2924 (vs, CH), 2854 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C51H86O5 (M + H+) 779.6548, found 779.6550.
3.1.33. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-decanoyl-3-docosahexaenoyl-sn-glycerol (9c)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-docosahexaenoyl-sn-glycerol 9 (106 mg, 0.162 mmol), decanoic acid (32 mg, 0.186 mmol), DMAP (23 mg, 0.188 mmol) and EDAC (47 mg, 0.245 mmol) in 1 mL CH2Cl2. The product 9c (120 mg, 0.149 mmol) was afforded as colorless oil, yield 92%. −7.7 (c 0.88, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 14H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.41–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.03 (m, 2H, =CCH2CH3), 2.03–1.98 (m, 4H, =CCH2 in selachyl), 1.65–1.58 (m, 2H, CH2CH2COO in SFA), 1.58–1.50 (m, 2H, OCH2CH2), 1.39–1.21 (m, 34H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.12 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9 (2), 30.9, 29.8 (2), 29.6, 29.5 (2), 29.4 (2), 29.3 (5), 29.1, 27.2 (2), 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2854 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C53H90O5 (M + NH4+) 824.7127, found 824.7128.
3.1.34. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-dodecanoyl-3-docosahexaenoyl-sn-glycerol (9d)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-docosahexaenoyl-sn-glycerol 9 (55 mg, 0.084 mmol), dodecanoic acid (18 mg, 0.090 mmol), DMAP (11 mg, 0.090 mmol) and EDAC (23 mg, 0.120 mmol) in 1 mL CH2Cl2. The product 9d (64 mg, 0.077 mmol) was afforded as pale yellow oil, yield 91%. −7.6 (c 1.0, benzene). 1H NMR (400 MHz, CDCl3) δ 5.43–5.28 (m, 14H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.79 (m, 10H, =CCH2C=), 2.39–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.03 (m, 2H, CCH2CH3), 2.03–1.98 (m, 4H, =CCH2 in selachyl), 1.65–1.57 (m, 2H, CH2CH2COO in SFA), 1.57–1.50 (m, 2H, OCH2CH2), 1.38–1.22 (m, 36H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.13 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9 (2), 30.9, 29.8 (2), 29.6 (3), 29.5 (3), 29.4, 29.3 (5), 29.1, 27.2 (2), 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C55H94O5 (M + H+) 835.7174, found 835.7161.
3.1.35. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-tetradecanoyl-3-docosahexaenoyl-sn-glycerol (9e)
The same procedure was followed as described for 4a using (R)-1-O-(Z)-octadec-9-enyl-3-docosahexaenoyl-sn-glycerol 9 (55 mg, 0.084 mmol), tetradecanoic acid (20 mg, 0.088 mmol), DMAP (7 mg, 0.059 mmol) and EDAC (27 mg, 0.141 mmol) in 1 mL CH2Cl2. The product 9e (67 mg, 0.078 mmol) was afforded as pale yellow oil, yield 92%. −8.0 (c 0.96, benzene). 1H NMR (400 MHz, CDCl3): δ 5.43–5.28 (m, 14H, =CH), 5.22–5.17 (m, 1H, CH2CHCH2), 4.35 (dd, 1H, J = 11.9 Hz, J = 3.7 Hz, CH2OCO), 4.17 (dd, 1H, J = 11.9 Hz, J = 6.5 Hz, CH2OCO), 3.57–3.50 (2xdd, 2H, J = 10.6 Hz, J = 5.3 Hz, CHCH2O), 3.47–3.37 (2xdt, 2H, J = 9.3 Hz, J = 6.6 Hz, OCH2CH2), 2.88–2.80 (m, 10H, =CCH2C=), 2.39–2.35 (m, 4H, CH2CH2COO in DHA), 2.32 (t, 2H, J = 7.5 Hz, CH2COO in SFA), 2.11–2.03 (m, 2H, =CCH2CH3), 2.03–1.98 (m, 4H, =CCH2 in selachyl), 1.65–1.56 (m, 2H, CH2CH2COO in SFA), 1.56–1.50 (m, 2H, OCH2CH2), 1.38–1.20 (m, 40H, CH2), 0.97 (t, 3H, J = 7.5 Hz, CH3 in DHA), 0.88 (t, 6H, J = 6.8 Hz, CH3) ppm; 13C NMR (CDCl3) δ 173.13 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9 (2), 29.8 (2), 29.7 (4), 29.6 (2), 29.5 (3), 29.4 (2), 29.3 (4), 29.1, 27.2 (2), 26.0, 25.6 (3), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm; IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1742 (vs, C=O) cm−1; HRMS m/z calcd. for C57H98O5 (M + H+) 863.7487, found 863.7490.
3.1.36. Synthesis of 1-O-(Z)-Octadec-9-enyl-2-hexadecanoyl-3-docosahexaenoyl-sn-glycerol (9f)
The same procedure was followed as described for
4a using (
R)-1-
O-(Z)-octadec-9-enyl-3-docosahexaenoyl-
sn-glycerol
9 (61 mg, 0.093 mmol), hexadecanoic acid (27 mg, 0.105 mmol), DMAP (10 mg, 0.082 mmol) and EDAC (25 mg, 0.130 mmol) in 1 mL CH
2Cl
2. The product
9f (74 mg, 0.083 mmol) was afforded as pale yellow oil, yield 89%.
−7.1 (
c 0.89, benzene).
1H NMR (400 MHz, CDCl
3) δ 5.43–5.28 (m, 14H, =C
H), 5.22–5.17 (m, 1H, CH
2C
HCH
2), 4.35 (dd, 1H,
J = 11.9 Hz,
J = 3.7 Hz, C
H2OCO), 4.17 (dd, 1H,
J = 11.9 Hz,
J = 6.5 Hz, C
H2OCO), 3.57–3.50 (2xdd, 2H,
J = 10.6 Hz,
J = 5.3 Hz, CHC
H2O), 3.47–3.37 (2xdt, 2H,
J = 9.3 Hz,
J = 6.6 Hz, OC
H2CH
2), 2.88–2.80 (m, 10H, =CC
H2C=), 2.41–2.35 (m, 4H, C
H2C
H2COO in DHA), 2.32 (t, 2H,
J = 7.5 Hz, C
H2COO in SFA), 2.11–2.03 (m, 2H, =CC
H2CH
3), 2.03–1.99 (m, 4H, =CC
H2 in selachyl), 1.65–1.58 (m, 2H, C
H2CH
2COO in SFA), 1.58–1.50 (m, 2H, OCH
2C
H2), 1.40–1.20 (m, 44H, C
H2), 0.97 (t, 3H,
J = 7.5 Hz, C
H3 in DHA), 0.88 (t, 6H,
J = 6.8 Hz, C
H3) ppm (
Supplementary Information, Figure S20);
13C NMR (CDCl
3) δ 173.12 (β, C=O in SFA), 172.68 (α, C=O in DHA), 132.0, 129.9, 129.8, 129.3, 128.6, 128.3 (2), 128.2, 128.1 (2), 128.0, 127.9, 127.8, 127.0, 71.8, 70.0, 69.0, 63.0, 34.3, 34.0, 31.9 (2), 30.9, 29.8 (2), 29.7 (4), 29.6 (2), 29.5 (3), 29.4 (2), 29.3 (4), 29.1, 27.2 (2), 26.0, 25.6 (4), 25.5, 25.0, 22.7 (3), 20.6, 14.3, 14.1 (2) ppm (
Supplementary Information, Figure S21); IR (ZnSe) 3013 (s, CH), 2923 (vs, CH), 2853 (s, CH), 1743 (vs, C=O) cm
−1; HRMS
m/z calcd. for C
59H
102O
5 (M + NH
4+) 908.8066, found 908.8088.