Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction

2. Results
2.1. Identification of Fucoxanthin and Fucoxanthinol by LC-MS/MS
| Analyte | Precursor Ion (m/z) | Daughter Ion (m/z) | Dwell Time (s) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Fucoxanthin | 659.3 | 109.0, 581.4, 641.4 | 0.2 | 48 | 10 | 28 | 18 |
| Fucoxanthinol | 617.2 | 109.0, 581.4, 599.4 | 0.2 | 15 | 11 | 28 | 8 |
| IS | 429.4 | 313.2 | 0.2 | 60 | 10 | 37 | 13 |




2.2. Method Validation
2.2.1. Selectivity and Specificity
2.2.2. Calibration Curve Linearity, Lower Limit of Quantification, and Limit of Detection
2.2.3. Accuracy and Precision
| Analyte | Concentration (ng/mL) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Precision (%) | Accuracy (%) | Mean ± SD (ng/mL) | Precision (%) | Accuracy (%) | ||
| Fucoxanthin | 11.25 | 12.63 ± 0.25 | 1.99 | 111.80 | 11.83 ± 0.84 | 7.09 | 104.72 |
| 90 | 96.80 ± 4.54 | 4.69 | 107.56 | 80.87 ± 2.34 | 2.89 | 89.96 | |
| 360 | 378.67 ± 21.23 | 5.74 | 105.19 | 312.00 ± 12.29 | 12.29 | 86.67 | |
| Fucoxanthinol | 9.4 | 10.42 ± 0.65 | 6.26 | 110.89 | 9.91 ± 0.48 | 4.84 | 105.46 |
| 75 | 81.93 ± 4.55 | 5.56 | 109.24 | 68.77 ± 0.58 | 0.84 | 91.69 | |
| 300 | 317.00 ± 8.72 | 2.75 | 105.67 | 265.33 ± 4.51 | 1.7 | 88.44 | |
2.2.4. Recovery and Matrix Effects
| Analyte | Concentration (ng/mL) | Recovery | Matrix Effects | ||
|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | RSD (%) | Mean ± SD (ng/mL) | RSD (%) | ||
| Fucoxanthin | 12.25 | 101.00 ± 3.61 | 3.61 | 86.92 ± 14.34 | 16.50 |
| 90.0 | 96.73 ± 7.62 | 7.88 | 87.71 ± 9.91 | 11.29 | |
| 360 | 92.90 ± 2.91 | 3.14 | 98.44 ± 9.32 | 9.46 | |
| Fucoxanthinol | 9.4 | 91.58 ± 1.84 | 2.01 | 85.90 ± 14.47 | 16.84 |
| 75 | 84.76 ± 2.44 | 2.88 | 87.59 ± 7.60 | 8.67 | |
| 300 | 88.43 ± 1.57 | 1.78 | 95.08 ± 7.51 | 7.90 | |
2.2.5. Stability
| Storage Condition (−80 °C) | Fucoxanthin | Fucoxanthinol | ||||
|---|---|---|---|---|---|---|
| Concentration (ng/mL) | Accuracy (%) | RSD (%) | Concentration (ng/mL) | Accuracy (%) | RSD (%) | |
| 1 freeze-thaw cycle | 12.25 | 82.55 | 4.03 | 9.4 | 99.60 | 4.83 |
| 90.0 | 87.40 | 1.78 | 75 | 89.05 | 5.00 | |
| 360 | 80.35 | 3.78 | 300 | 89.45 | 4.03 | |
| 2 freeze-thaw cycles | 12.25 | 102.20 | 6.64 | 9.4 | 101.90 | 12.63 |
| 90.0 | 80.35 | 0.44 | 75 | 91.30 | 8.83 | |
| 360 | 86.75 | 3.67 | 300 | 91.75 | 8.25 | |
| 3 freeze-thaw cycles | 12.25 | 113.0 | 7.51 | 9.4 | 118.0 | 5.52 |
| 90.0 | 90.55 | 3.36 | 75 | 99.70 | 6.10 | |
| 360 | 85.95 | 1.89 | 300 | 98.60 | 0.14 | |
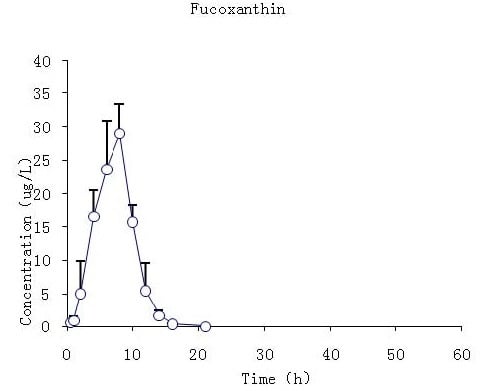
2.3. Pharmacokinetic Studies of Fucoxanthin and Fucoxanthinol
2.3.1. Studies of i.v. Fucoxanthin Administration

| Parameters | Unit | Fucoxanthin | Fucoxanthinol | ||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| Cmax | μg/L | 598.2 | 64.8 | ||
| Tmax | h | 1.0 | 0.4 | ||
| AUC0–t | μg·h/L | 9861.8 | 749.0 | 3260.6 | 326.1 |
| AUC0–∞ | μg·h/L | 9871.0 | 746.9 | 3954.7 | 354.8 |
| t1/2 | h | 2.3 | 0.8 | 11.9 | 2.2 |
| CL | L/h/kg | 0.2 | 0.0 | 0.5 | 0.0 |
| Vd | L/kg | 0.7 | 0.3 | 8.8 | 2.0 |
2.3.2. Studies of i.g. Fucoxanthin Administration

| Parameters | Unit | Fucoxanthin | Fucoxanthinol | ||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| Cmax | μg/L | 29.1 | 4.4 | 263.3 | 93.9 |
| Tmax | h | 7.7 | 0.8 | 11 | 1.1 |
| AUC0–t | μg·h/L | 191.1 | 39.6 | 5017.6 | 1371.0 |
| AUC0–∞ | μg·h/L | 195.1 | 42.2 | 5304.2 | 1266.9 |
| t1/2 | h | 1.2 | 0.6 | 9.3 | 5.8 |
| CL | L/h/kg | 347.8 | 81.8 | 12.8 | 3.1 |
| Vd | L/kg | 550.2 | 226.1 | 181.5 | 142.4 |
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Chromatographic and Mass Spectrometric Conditions
4.2.1. Liquid Chromatography Conditions
4.2.2. Mass Spectrometric Conditions
4.3. Stock and Working Solutions
4.4. Calibration Standard Curves and QC Samples
4.5. Plasma Sample Preparation
4.6. Method Validation
4.7. Stability
4.8. Pharmacokinetic and Bioavailability Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Kil, N.K.; Soo, J.H.; Sung, M.K.; Ginnae, A.; You, J.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. Vitr. 2010, 24, 1648–1654. [Google Scholar]
- Koyagagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Himeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta 2005, 1762, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.H.; Weon, J.Y.; Kil, N.K.; Gin, N.A.; Sung, M.K.; Do, H.K. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, M.K.; Park, Y.B.; Shin, Y.C.; Choi, M.S. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem. Toxicol. 2011, 49, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skinphotoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast andcolon cancer cells. Comp. Biochem. Phys. C 2006, 142, 53–59. [Google Scholar]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.N.; Taira, N.; et al. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M. Radical scavenging and singlet oxygenquenching activity of marine carotenoid fucoxanthin and itsmetabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H. Biotransformation offucoxanthinol into amarouciaxanthin A in mice and HepG2cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tatsuya, S.; Vallikannan, B.; Wakako, T.; Akihiko, N. Brown Algae Fucoxanthin Is Hydrolyzed to Fucoxanthinol during Absorptionby Caco-2 Human Intestinal Cells and Mice. Nutr. Metab. 2002, 132, 946–951. [Google Scholar]
- Takashi, H.; Yoshiaki, O.; Mayuko, T.; Swadesh, K.D.; Masashi, M.; Kazuto, Y.; Takashi, M.; Kazuki, K. The distribution and accumulation of fucoxanthin and its metabolites afteroral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar]
- Eiichi, K.N.; Tatsuya, S.W.; Akihiko, N. Antiproliferative effect of neoxanthin and fucoxanthin oncultured cells. Fish. Sci. 2005, 71, 459–461. [Google Scholar]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, H.; Wen, H.; Fang, H.; Hong, Z.; Yi, R.; Liu, R. Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 6521-6536. https://doi.org/10.3390/md13106521
Zhang Y, Wu H, Wen H, Fang H, Hong Z, Yi R, Liu R. Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Marine Drugs. 2015; 13(10):6521-6536. https://doi.org/10.3390/md13106521
Chicago/Turabian StyleZhang, Yiping, Hao Wu, Hongmei Wen, Hua Fang, Zhuan Hong, Ruizao Yi, and Rui Liu. 2015. "Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry" Marine Drugs 13, no. 10: 6521-6536. https://doi.org/10.3390/md13106521
APA StyleZhang, Y., Wu, H., Wen, H., Fang, H., Hong, Z., Yi, R., & Liu, R. (2015). Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Marine Drugs, 13(10), 6521-6536. https://doi.org/10.3390/md13106521