Distribution in Different Organisms of Amino Acid Oxidases with FAD or a Quinone As Cofactor and Their Role as Antimicrobial Proteins in Marine Bacteria
Abstract
:1. Introduction

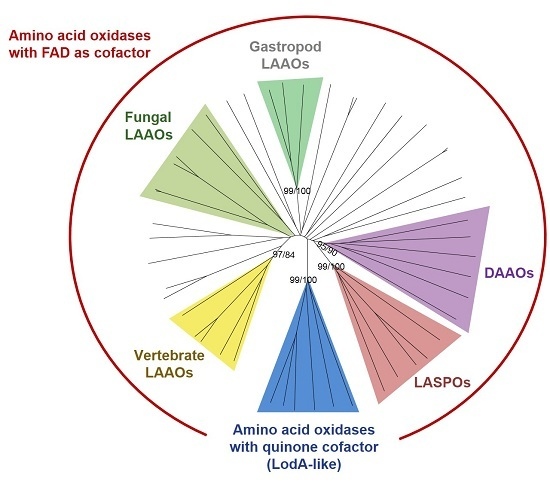
2. Phylogenetic Analysis of Proteins with Amino Acid Oxidase Activity

2.1. AAOs with a Quinone Cofactor (LodA-Like Proteins)
2.2. d-Amino Acid Oxidases
2.3. l-Aspartate Oxidases

2.4. LAAOs in Animals
2.4.1. LAAOs from Vertebrates
2.4.2. LAAOs in Gastropods
2.5. Fungal LAAOs

2.6. Other Bacterial LAAOs
3. Amino Acid Oxidases with Antimicrobial Activity in Marine Bacteria
Supplementary Files
Supplementary File 1Conflicts of Interest
References
- Izidoro, L.F.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.; et al. Snake venom l-amino acid oxidases: Trends in pharmacology and biochemistry. Biomed. Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Qiao, H. Advances in non-snake venom l-amino acid oxidase. Appl. Biochem. Biotechnol. 2012, 167, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Molla, G.; Sacchi, S.; Rosini, E.; Verga, R.; Pilone, M. Properties and applications of microbial d-amino acid oxidases: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 78, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Molla, G. New biotech applications from evolved d-amino acid oxidases. Trends Biotechnol. 2011, 29, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Settembre, E.C.; Dorrestein, P.C.; Park, J.H.; Augustine, A.M.; Begley, T.P.; Ealick, S.E. Structural and mechanistic studies on ThiO, a glycine oxidase essential for thiamin biosynthesis in Bacillus subtilis. Biochemistry 2003, 42, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Lucas-Elio, P.; Sanchez-Amat, A.; Solano, F. A novel type of lysine oxidase: l-Lysine-epsilon-oxidase. Biochim. Biophys. Acta 2006, 1764, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Elio, P.; Gomez, D.; Solano, F.; Sanchez-Amat, A. The antimicrobial activity of marinocine, synthesized by Marinomonas mediterranea, is due to hydrogen peroxide generated by its lysine oxidase activity. J. Bacteriol. 2006, 188, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Verdu, M.; Gomez, D.; Solano, F.; Lucas-Elio, P.; Sanchez-Amat, A. LodB is required for the recombinant synthesis of the quinoprotein l-lysine-ε-oxidase from Marinomonas mediterranea. Appl. Microbiol. Biotechnol. 2014, 98, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Nakano, S.; Matsui, D.; Akaji, S.; Inagaki, K.; Asano, Y. X-ray crystallographic evidence for the presence of the cysteine tryptophylquinone cofactor in l-lysine epsilon-oxidase from Marinomonas mediterranea. J. Biochem. 2013, 154, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Brocal, J.C.; Lucas-Elio, P.; Sanchez-Amat, A. Identification in Marinomonas mediterranea of a novel quinoprotein with glycine oxidase activity. Microbiologyopen 2013, 2, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Verdu, M.; Campillo-Brocal, J.C.; Lucas-Elio, P.; Davidson, V.L.; Sanchez-Amat, A. Characterization of recombinant biosynthetic precursors of the cysteine tryptophylquinone cofactors of l-lysine-epsilon-oxidase and glycine oxidase from Marinomonas mediterranea. Biochim. Biophys. Acta 2015, 1854, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Campillo-Brocal, J.C.; Chacon-Verdu, M.D.; Lucas-Elío, P.; Sanchez-Amat, A. Distribution in microbial genomes of genes similar to lodA and goxA which encode a novel family of quinoproteins with amino acid oxidase activity. BMC Genomics 2015, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Lucas-Elio, P.; Egan, S.; Thomas, T.; Webb, J.S.; Sanchez-Amat, A.; Kjelleberg, S. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several Gram-negative bacteria. J. Bacteriol. 2008, 190, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.A.; Cheng, C.H.; Lo, C.T.; Liu, S.Y.; Lee, J.W.; Peng, K.C. A novel l-amino acid oxidase from Trichoderma harzianum ETS 323 associated with antagonism of Rhizoctonia solani. J. Agric. Food Chem. 2011, 59, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Kitani, Y.; Kikuchi, N.; Zhang, G.; Ishizaki, S.; Shimakura, K.; Shiomi, K.; Nagashima, Y. Antibacterial action of l-amino acid oxidase from the skin mucus of rockfish Sebastes schlegelii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Puiffe, M.L.; Lachaise, I.; Molinier-Frenkel, V.R.; Castellano, F. Antibacterial properties of the mammalian l-amino acid oxidase IL4I1. PLoS ONE 2013, 8, e54589. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Motta, P.; Molla, G. l-Amino acid oxidase as biocatalyst: A dream too far? Appl. Microbiol. Biotechnol. 2013, 97, 9323–9341. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Ishikawa, T.; Nakamura, T.; Miura, T. Antibacterial properties of l-amino acid oxidase: Mechanisms of action and perspectives for therapeutic applications. Appl. Microbiol. Biotechnol. 2015, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; Pollegioni, L.; Ghisla, S.; Orru, R.; Molla, G. Optimization of d-amino acid oxidase for low substrate concentrations—Towards a cancer enzyme therapy. FEBS J. 2009, 276, 4921–4932. [Google Scholar] [CrossRef] [PubMed]
- Matsui, D.; Im, D.H.; Sugawara, A.; Fukuta, Y.; Fushinobu, S.; Isobe, K.; Asano, Y. Mutational and crystallographic analysis of l-amino acid oxidase/monooxygenase from Pseudomonas sp. AIU 813: Interconversion between oxidase and monooxygenase activities. FEBS Open Bio. 2014, 4, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Chen, W.; Shi, W.; Qi, F.; Dong, X. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J. Bacteriol. 2008, 190, 4716–4721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, L.; Tong, H.; Dong, X. Role of operon aaoSo-mutT in antioxidant defense in Streptococcus oligofermentans. PLoS ONE 2012, 7, e38133. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sehanobish, E.; Chacon-Verdu, M.D.; Sanchez-Amat, A.; Davidson, V.L. Roles of active site residues in LodA, a cysteine tryptophylquinone dependent epsilon-lysine oxidase. Arch. Biochem. Biophys. 2015, 579, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Abe, K.; Kera, Y. Bacterial d-amino acid oxidases: Recent findings and future perspectives. Bioengineered 2015, 6, 237–241. [Google Scholar] [CrossRef] [PubMed]
- D'Aniello, A.; Vetere, A.; Petrucelli, L. Further study on the specificity of d-amino acid oxidase and of d-aspartate oxidase and time course for complete oxidation of d-amino acids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1993, 105, 731–734. [Google Scholar] [CrossRef]
- Takahashi, S.; Furukawara, M.; Omae, K.; Tadokoro, N.; Saito, Y.; Abe, K.; Kera, Y. A highly stable d-amino acid oxidase of the thermophilic bacterium Rubrobacter xylanophilus. Appl. Environ. Microbiol. 2014, 80, 7219–7229. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; DArrigo, P.; Rosini, E.; Pedrocchi-Fantoni, G.; Tessaro, D.; Molla, G.; Servi, S.; Pollegioni, L. Activity of yeast d-amino acid oxidase on aromatic unnatural amino acids. J. Mol. Catal. B Enzym. 2008, 50, 93–98. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Kohnehrouz, B.B. Molecular cloning and expression in Escherichia coli of an active fused Zea mays L. d-amino acid oxidase. Biochemistry (Mosc.) 2009, 74, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Katane, M.; Saitoh, Y.; Seida, Y.; Sekine, M.; Furuchi, T.; Homma, H. Comparative characterization of three d-aspartate oxidases and one d-amino acid oxidase from Caenorhabditis elegans. Chem. Biodivers. 2010, 7, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Sarower, G.M.; Okada, S.; Abe, H. Molecular characterization of d-amino acid oxidase from common carp Cyprinus carpio and its induction with exogenous free d-alanine. Arch. Biochem. Biophys. 2003, 420, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Molla, G.; Sacchi, S.; Bernasconi, M.; Pilone, M.S.; Fukui, K.; Pollegioni, L. Characterization of human d-amino acid oxidase. FEBS Lett. 2006, 580, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Piubelli, L.; Sacchi, S.; Pilone, M.S.; Molla, G. Physiological functions of d-amino acid oxidases: From yeast to humans. Cell. Mol. Life Sci. 2007, 64, 1373–1394. [Google Scholar] [CrossRef] [PubMed]
- Khoronenkova, S.V.; Tishkov, V.I. d-Amino acid oxidase: Physiological role and applications. Biochemistry (Mosc) 2008, 73, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Nasu, S.; Wicks, F.D.; Gholson, R.K. l-Aspartate oxidase, a newly discovered enzyme of Escherichia coli, is the B protein of quinolinate synthetase. J. Biol. Chem. 1982, 257, 626–632. [Google Scholar] [PubMed]
- Korshunov, S.; Imlay, J.A. Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol. Microbiol. 2010, 75, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Savchenko, A.; Yakunin, A.; Zhang, R.; Edwards, A.; Arrowsmith, C.; Tong, L. Aspartate dehydrogenase, a novel enzyme identified from structural and functional studies of TM1643. J. Biol. Chem. 2003, 278, 8804–8808. [Google Scholar] [CrossRef] [PubMed]
- Bossi, R.T.; Negri, A.; Tedeschi, G.; Mattevi, A. Structure of FAD-bound l-aspartate oxidase: Insight into substrate specificity and catalysis. Biochemistry 2002, 41, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, A.; Tedeschi, G.; Bacchella, L.; Coda, A.; Negri, A.; Ronchi, S. Structure of l-aspartate oxidase: Implications for the succinate dehydrogenase/fumarate reductase oxidoreductase family. Structure 1999, 7, 745–756. [Google Scholar] [CrossRef]
- Tedeschi, G.; Nonnis, S.; Strumbo, B.; Cruciani, G.; Carosati, E.; Negri, A. On the catalytic role of the active site residue E121 of E. coli l-aspartate oxidase. Biochimie 2010, 92, 1335–1342. [Google Scholar]
- Sakuraba, H.; Satomura, T.; Kawakami, R.; Yamamoto, S.; Kawarabayasi, Y.; Kikuchi, H.; Ohshima, T. l-Aspartate oxidase is present in the anaerobic hyperthermophilic archaeon Pyrococcus horikoshii OT-3: Characteristics and role in the de novo biosynthesis of nicotinamide adenine dinucleotide proposed by genome sequencing. Extremophiles 2002, 6, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, I.; Nonnis, S.; Monteferrante, C.; Heathcote, P.; Hartig, E.; Bottger, L.H.; Trautwein, A.X.; Negri, A.; Albertini, A.M.; Tedeschi, G. Characterization of l-aspartate oxidase and quinolinate synthase from Bacillus subtilis. FEBS J. 2008, 275, 5090–5107. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, D.; Pollegioni, L.; Tessaro, D.; Servi, S.; Molla, G. A thermostable l-aspartate oxidase: A new tool for biotechnological applications. Appl. Microbiol. Biotechnol. 2013, 97, 7285–7295. [Google Scholar] [CrossRef] [PubMed]
- Mutaguchi, Y.; Ohmori, T.; Sakuraba, H.; Yoneda, K.; Doi, K.; Ohshima, T. Visible wavelength spectrophotometric assays of l-aspartate and d-aspartate using hyperthermophilic enzyme systems. Anal. Biochem. 2011, 409, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Kwan, A.L.; Dutcher, S.K. Synthesizing and salvaging NAD: Lessons learned from Chlamydomonas reinhardtii. PLoS Genet. 2010, 6, e1001105. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.L.; Fioravanti, R.; Barbabella, G.; Prosseda, G.; Colonna, B.; Casalino, M. Molecular evolution of the nicotinic acid requirement within the Shigella/EIEC pathotype. Int. J. Med. Microbiol. 2013, 303, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. Origin and diversification of the l-amino oxidase family in innate immune defenses of animals. Immunogenetics 2010, 62, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Clemetson, K.J. Snake venom l-amino acid oxidases. Toxicon 2002, 40, 659–665. [Google Scholar] [CrossRef]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.C.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Tsukamoto, C.; Kitani, Y.; Ishizaki, S.; Nagai, H.; Yanagimoto, T. Isolation and cDNA cloning of an antibacterial l-amino acid oxidase from the skin mucus of the great sculpin Myoxocephalus polyacanthocephalus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Hayashi, Y.; Kitani, Y.; Ren, H.; Hayashi, T.; Nagashima, Y. Optical enzyme sensor for determining l-lysine content using l-lysine oxidase from the rockfish Sebastes schlegeli. Anal. Bioanal. Chem. 2008, 391, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Kitani, Y.; Ishida, M.; Ishizaki, S.; Nagashima, Y. Discovery of serum l-amino acid oxidase in the rockfish Sebastes schlegeli: Isolation and biochemical characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 157, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, R.; Xie, M.; Li, A. The serum of rabbitfish (Siganus oramin) has antimicrobial activity to some pathogenic organisms and a novel serum l-amino acid oxidase is isolated. Fish. Shellfish Immunol. 2011, 30, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Kitani, Y.; Fernandes, J.M.O.; Kiron, V. Identification of the atlantic cod l-amino acid oxidase and its alterations following bacterial exposure. Dev. Comp. Immunol. 2015, 50, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Boulland, M.L.; Marquet, J.; Molinier-Frenkel, V.R.; Moller, P.; Guiter, C.; Lasoudris, F.; Copie-Bergman, C.; Baia, M.; Gaulard, P.; Leroy, K.; et al. Human IL4I1 is a secreted l-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood 2007, 110, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nonobe, E.; Kobayashi, Y.; Kuraishi, T.; Aoki, F.; Yamamoto, K.; Sakai, S. Characterization and expression of l-amino acid oxidase of mouse milk. J. Biol. Chem. 2002, 277, 19080–19086. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Kicklighter, C.E.; Schmidt, M.; Kamio, M.; Yang, H.; Elkin, D.; Michel, W.C.; Tai, P.C.; Derby, C.D. Packaging of chemicals in the defensive secretory glands of the sea hare Aplysia californica. J. Exp. Biol. 2006, 209, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Johnson, P.M.; Ko, K.C.; Kamio, M.; Germann, M.W.; Derby, C.D.; Tai, P.C. Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing l-amino acid oxidase from ink of the sea hare Aplysia californica. J. Exp. Biol. 2005, 208, 3609–3622. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.C.; Wang, B.; Tai, P.C.; Derby, C.D. Identification of potent bactericidal compounds produced by escapin, an l-amino acid oxidase in the ink of the sea hare Aplysia californica. Antimicrob. Agents Chemother. 2008, 52, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.C.; Tai, P.C.; Derby, C.D. Mechanisms of action of escapin, a bactericidal agent in the ink secretion of the sea hare Aplysia californica: Rapid and long-lasting DNA condensation and involvement of the OxyR-regulated oxidative stress pathway. Antimicrob. Agents Chemother. 2012, 56, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Butzke, D.; Hurwitz, R.; Thiede, B.; Goedert, S.; Rudel, T. Cloning and biochemical characterization of APIT, a new l-amino acid oxidase from Aplysia punctata. Toxicon 2005, 46, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Ehara, T.; Kitajima, S.; Kanzawa, N.; Tamiya, T.; Tsuchiya, T. Antimicrobial action of achacin is mediated by l-amino acid oxidase activity. FEBS Lett. 2002, 531, 509–512. [Google Scholar] [CrossRef]
- Kanzawa, N.; Shintani, S.; Ohta, K.; Kitajima, S.; Ehara, T.; Kobayashi, H.; Kizaki, H.; Tsuchiya, T. Achacin induces cell death in HeLa cells through two different mechanisms. Arch. Biochem. Biophys. 2004, 422, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alegre, M.; Franco, A.R. Resistance to l-methionine-S-sulfoximine in Chlamydomonas reinhardtii is due to an alteration in a general amino acid transport system. Planta 1998, 207, 20–26. [Google Scholar] [CrossRef]
- Vallon, O.; Bulte, L.; Kuras, R.; Olive, J.; Wollman, F.A. Extensive accumulation of an extracellular l-amino-acid oxidase during gametogenesis of Chlamydomonas reinhardtii. Eur. J. Biochem. 1993, 215, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Askin, M.C.; Hynes, M.J. Amino acid catabolism by an areA-regulated gene encoding an l-amino acid oxidase with broad substrate specificity in Aspergillus nidulans. Appl. Environ. Microbiol. 2005, 71, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Sikora, L.; Marzluf, G.A. Regulation of l-amino acid oxidase and of d-amino acid oxidase in Neurospora crassa. Mol. Gen. Genet. 1982, 186, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, J.T.; Timoneni, S. Identification of nitrogen mineralization enzymes, l-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol. Res. 2008, 112, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gogoi, B.K.; Bezbaruah, R.L. Optimization of medium and cultivation conditions for l-amino acid oxidase production by Aspergillus fumigatus. Can. J. Microbiol. 2009, 55, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gogoi, B.; Bezbaruah, R. Racemic resolution of some dl-amino acids using Aspergillus fumigatus l-amino acid oxidase. Curr. Microbiol. 2011, 63, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, H.; Kodama, K.; Kuninaka, A.; Yoshino, H.; Misono, H.; Soda, K. A new antitumor enzyme, l-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J. Biol. Chem. 1980, 255, 976–981. [Google Scholar] [PubMed]
- Amano, M.; Mizuguchi, H.; Sano, T.; Kondo, H.; Shinyashiki, K.; Inagaki, J.; Tamura, T.; Kawaguchi, T.; Kusakabe, H.; Imada, K.; et al. Recombinant expression, molecular characterization and crystal structure of antitumor enzyme, l-lysine-α-oxidase from Trichoderma viride. J. Biochem. 2015, 157, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Lukasheva, E.V.; Berezov, T.T. l-Lysine alpha-oxidase: Physicochemical and biological properties. Biochemistry (Mosc) 2002, 67, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Narang, J.; Sunny; Pundir, C.S. Immobilization of lysine oxidase on a gold-platinum nanoparticles modified Au electrode for detection of lysine. Enzyme Microb. Technol. 2013, 52, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Stamm, W.W.; Kusakabe, H.; Kula, M.R. Enzymatic determination of l-lysine by flow-injection techniques. Anal. Chim. Acta 1990, 235, 329–335. [Google Scholar] [CrossRef]
- Yang, C.A.; Cheng, C.H.; Liu, S.Y.; Lo, C.T.; Lee, J.W.; Peng, K.C. Identification of antibacterial mechanism of l-amino acid oxidase derived from Trichoderma harzianum ETS 323. FEBS J. 2011, 278, 3381–3394. [Google Scholar] [CrossRef] [PubMed]
- Akyilmaz, E.; Erdogan, A.; Ozturk, R.; Yasa, I. Sensitive determination of l-lysine with a new amperometric microbial biosensor based on Saccharomyces cerevisiae yeast cells. Biosens. Bioelectron. 2007, 22, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Sawada, H.; Hirahara, T.; Ito, K.; Ohshiro, T.; Izumi, Y. A novel enzyme, l-tryptophan oxidase, from a basidiomycete, Coprinus sp. SF-1: Purification and characterization. Biosci. Biotechnol. Biochem. 2000, 64, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, Y.; Imanaka, T. Purification and characterization of a novel glycine oxidase from Bacillus subtilis. FEBS Lett. 1998, 438, 263–266. [Google Scholar] [CrossRef]
- Job, V.; Marcone, G.L.; Pilone, M.S.; Pollegioni, L. Glycine oxidase from Bacillus subtilis. Characterization of a new flavoprotein. J. Biol. Chem. 2002, 277, 6985–6993. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, I.; Navarro-Fernandez, J.; Garcia-Carmona, F.; Takami, H.; Sanchez-Ferrer, A. Characterization and structural modeling of a novel thermostable glycine oxidase from Geobacillus kaustophilus HTA426. Proteins 2008, 70, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; Piubelli, L.; Molla, G.; Frattini, L.; Valentino, M.; Varriale, A.; D'Auria, S.; Pollegioni, L. Novel biosensors based on optimized glycine oxidase. FEBS J. 2014, 281, 3460–3472. [Google Scholar] [CrossRef] [PubMed]
- Nicolia, A.; Ferradini, N.; Molla, G.; Biagetti, E.; Pollegioni, L.; Veronesi, F.; Rosellini, D. Expression of an evolved engineered variant of a bacterial glycine oxidase leads to glyphosate resistance in alfalfa. J. Biotechnol. 2014, 184, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Lin, Y.; Wu, G.; Lu, Y.; Zhan, T.; Kumar, A.; Zhang, L.; Liu, Z. Improvement of glycine oxidase by DNA shuffling, and site-saturation mutagenesis of F247 residue. Int. J. Biol. Macromol. 2015, 79, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Trickey, P.; Wagner, M.A.; Jorns, M.S.; Mathews, F.S. Monomeric sarcosine oxidase: Structure of a covalently flavinylated amine oxidizing enzyme. Structure 1999, 7, 331–345. [Google Scholar] [CrossRef]
- Boggs, J.M.; South, A.H.; Hughes, A.L. Phylogenetic analysis supports horizontal gene transfer of l-amino acid oxidase gene in Streptococcus oligofermentans. Infect. Genet. Evol. 2012, 12, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Molla, G.; Nardini, M.; Motta, P.; D'Arrigo, P.; Panzeri, W.; Pollegioni, L. Aminoacetone oxidase from Streptococcus oligofermentans belongs to a new three-domain family of bacterial flavoproteins. Biochem. J. 2014, 464, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Swaving, J.; de Bont, J.A.; Westphal, A.; de, K.A. A novel type of pyridine nucleotide-disulfide oxidoreductase is essential for NAD+- and NADPH-dependent degradation of epoxyalkanes by Xanthobacter strain Py2. J. Bacteriol. 1996, 178, 6644–6646. [Google Scholar] [PubMed]
- delCardayre, S.B.; Davies, J.E. Staphylococcus aureus coenzyme A disulfide reductase, a new subfamily of pyridine nucleotide-disulfide oxidoreductase. Sequence, expression, and analysis of cdr. J. Biol. Chem. 1998, 273, 5752–5757. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wesener, S.R.; Zhang, H.; Cheng, Y.Q. An FAD-dependent pyridine nucleotide-disulfide oxidoreductase is involved in disulfide bond formation in FK228 anticancer depsipeptide. Chem. Biol. 2009, 16, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B.; Hummel, W.; Bommarius, A. l-Amino Acid Oxidase from Rhodococcus Species. U.S. Patent 6461841 B2, 8 October 2002. [Google Scholar]
- Faust, A.; Niefind, K.; Hummel, W.; Schomburg, D. The structure of a bacterial l-amino acid oxidase from Rhodococcus opacus gives new evidence for the hydride mechanism for dehydrogenation. J. Mol. Biol. 2007, 367, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Satou, S.; Matsumoto, E.; Yoshida, S.; Yamada, M.; Hibi, M.; Ogawa, J. Characterization and application of a l-specific amino acid oxidase from Rhodococcus sp. AIU LAB-3. J. Biosci. Bioeng. 2013, 115, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Brearley, G.M.; Price, C.P.; Atkinson, T.; Hammond, P.M. Purification and partial characterisation of a broad-range l-amino acid oxidase from Bacillus carotarum 2Pfa isolated from soil. Appl. Microbiol. Biotechnol. 1994, 41, 670–676. [Google Scholar] [CrossRef]
- Braun, M.; Kim, J.M.; Schmid, R.D. Purification and some properties of an extracellular l-amino acid oxidase from Cellulomonas cellulans AM8 isolated from soil. Appl. Microbiol. Biotechnol. 1992, 37, 594–598. [Google Scholar] [CrossRef]
- Bouvrette, P.; Luong, J.H. T. Purification and further characterization of an l-phenylalanine oxidase from Morganella morganii. Appl. Biochem. Biotechnol. 1994, 48, 61–74. [Google Scholar] [CrossRef]
- Coudert, M. Charcterization and physiological function of a soluble l-amino acid oxidase in Corynebacterium. Arch. Microbiol. 1975, 102, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Arima, J.; Sasaki, C.; Sakaguchi, C.; Mizuno, H.; Tamura, T.; Kashima, A.; Kusakabe, H.; Sugio, S.; Inagaki, K. Structural characterization of l-glutamate oxidase from Streptomyces sp. X-119–6. FEBS J. 2009, 276, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Arima, J.; Tamura, T.; Kusakabe, H.; Ashiuchi, M.; Yagi, T.; Tanaka, H.; Inagaki, K. Recombinant expression, biochemical characterization and stabilization through proteolysis of an l-glutamate oxidase from Streptomyces sp. X-119–6. J. Biochem. 2003, 134, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Sugawara, A.; Domon, H.; Fukuta, Y.; Asano, Y. Purification and characterization of an l-amino acid oxidase from Pseudomonas sp. AIU 813. J. Biosci. Bioeng. 2012, 114, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H. Oxidation and oxygenation of l-amino acids catalyzed by a l-phenylalanine oxidase (Deaminating and decarboxylating) from Pseudomonas Sp. P-501. J. Biochem. 1984, 96, 421–427. [Google Scholar] [PubMed]
- Suzuki, H.; Higashi, Y.; Asano, M.; Suguro, M.; Kigawa, M.; Maeda, M.; Katayama, S.; Mukouyama, E.B.; Uchiyama, K. Sequencing and expression of the l-phenylalanine oxidase gene from Pseudomonas sp. P-501. Proteolytic activation of the proenzyme. J. Biochem. 2004, 136, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, P.; Fitzpatrick, P.F. Analysis of the role of the active site residue Arg98 in the flavoprotein tryptophan 2-monooxygenase, a member of the l-amino oxidase family. Biochemistry 2003, 42, 13826–13832. [Google Scholar] [CrossRef] [PubMed]
- Genet, R.; Benetti, P.H.; Hammadi, A.; Menez, A. l-Tryptophan 2′,3′-oxidase from Chromobacterium violaceum. Substrate specificity and mechanistic implications. J. Biol. Chem. 1995, 270, 23540–23545. [Google Scholar] [CrossRef] [PubMed]
- Kameya, M.; Onaka, H.; Asano, Y. Selective tryptophan determination using tryptophan oxidases involved in bis-indole antibiotic biosynthesis. Anal. Biochem. 2013, 438, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Aldrich, C.; Sherman, D.H. Molecular analysis of the rebeccamycin l-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243. J. Bacteriol. 2005, 187, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Gau, A.E.; Heindl, A.F.; Nodop, A.F.; Kahmann, U.F.; Pistorius, E.K. l-Amino acid oxidases with specificity for basic l-amino acids in cyanobacteria. Z. Naturforsch. C Biosci. 2007, 62, 273–284. [Google Scholar] [CrossRef]
- Bockholt, R.; Scholten-Beck, G.F.; Pistorius, E.K. Construction and partial characterization of an l-amino acid oxidase-free Synechococcus PCC 7942 mutant and localization of the l-amino acid oxidase in the corresponding wild type. Biochim. Biophys. Acta 1996, 1307, 111–121. [Google Scholar] [CrossRef]
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, C.; Egan, S.; Franks, A.; McCloy, S.; Kjelleberg, S. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 2002, 41, 47–58. [Google Scholar] [CrossRef]
- Shikuma, N.J.; Pilhofer, M.; Weiss, G.L.; Hadfield, M.G.; Jensen, G.J.; Newman, D.K. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 2014, 343, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Celdran, D.; Espinosa, E.; Sanchez-Amat, A.; Atucha, A. Effects of epibiotic bacteria on leaf growth and epiphytes of seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2012, 456, 21–27. [Google Scholar] [CrossRef]
- James, S.G.; Holmstrom, C.; Kjelleberg, S. Purification and characterization of a novel antibacterial protein from the marine bacterium D2. Appl. Environ. Microbiol. 1996, 62, 2783–2788. [Google Scholar] [PubMed]
- Lucas-Elio, P.; Hernandez, P.; Sanchez-Amat, A.; Solano, F. Purification and partial characterization of marinocine, a new broad-spectrum antibacterial protein produced by Marinomonas mediterranea. Biochim. Biophys. Acta 2005, 1721, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, J.; Tang, K.; Shi, X.; Wang, S.; Zhu, W.M.; Zhang, X.H. Purification and characterization of antibacterial compounds of Pseudoalteromonas flavipulchra JG1. Microbiology 2012, 158, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Lin, C.Y.; Chen, C.A.; Wang, J.T.; Sheu, S.Y. Involvement of an l-amino acid oxidase in the activity of the marine bacterium Pseudoalteromonas flavipulchra against methicillin-resistant Staphylococcus aureus. Enzyme Microb. Technol. 2010, 47, 52–58. [Google Scholar] [CrossRef]
- Gomez, D.; Espinosa, E.; Bertazzo, M.; Lucas-Elio, P.; Solano, F.; Sanchez-Amat, A. The macromolecule with antimicrobial activity synthesized by Pseudoalteromonas luteoviolacea strains is an l-amino acid oxidase. Appl. Microbiol. Biotechnol. 2008, 79, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, N.; Qiao, H.; Qiu, J. Identification, cloning, and expression of l-amino acid oxidase from marine Pseudoalteromonas sp. B3. Sci. World J. 2014, 2014, 979858. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, J.; Lin, J.; Zhao, M.; Qiu, J. Exploring regulation genes involved in the expression of l-amino acid oxidase in Pseudoalteromonas sp. Rf-1. PLoS ONE 2015, 10, e0122741. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Lin, C.Y.; Sheu, S.Y. Investigating antimicrobial activity in Rheinheimera sp. due to hydrogen peroxide generated by l-lysine oxidase activity. Enzyme Microb. Technol. 2010, 46, 487–493. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campillo-Brocal, J.C.; Lucas-Elío, P.; Sanchez-Amat, A. Distribution in Different Organisms of Amino Acid Oxidases with FAD or a Quinone As Cofactor and Their Role as Antimicrobial Proteins in Marine Bacteria. Mar. Drugs 2015, 13, 7403-7418. https://doi.org/10.3390/md13127073
Campillo-Brocal JC, Lucas-Elío P, Sanchez-Amat A. Distribution in Different Organisms of Amino Acid Oxidases with FAD or a Quinone As Cofactor and Their Role as Antimicrobial Proteins in Marine Bacteria. Marine Drugs. 2015; 13(12):7403-7418. https://doi.org/10.3390/md13127073
Chicago/Turabian StyleCampillo-Brocal, Jonatan C., Patricia Lucas-Elío, and Antonio Sanchez-Amat. 2015. "Distribution in Different Organisms of Amino Acid Oxidases with FAD or a Quinone As Cofactor and Their Role as Antimicrobial Proteins in Marine Bacteria" Marine Drugs 13, no. 12: 7403-7418. https://doi.org/10.3390/md13127073
APA StyleCampillo-Brocal, J. C., Lucas-Elío, P., & Sanchez-Amat, A. (2015). Distribution in Different Organisms of Amino Acid Oxidases with FAD or a Quinone As Cofactor and Their Role as Antimicrobial Proteins in Marine Bacteria. Marine Drugs, 13(12), 7403-7418. https://doi.org/10.3390/md13127073