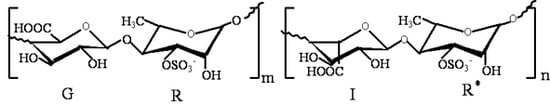
The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
2.1.1. Body Weight and Food Intakes



2.1.2. Lipid Content in Serum
2.1.3. FXR mRNA Expression
| Group | Dose (mg/kg) | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
|---|---|---|---|---|---|
| Male | |||||
| Normal control | - | 1.69 ± 0.15 | 0.87 ± 0.13 | 0.49 ± 0.03 | 1.04 ± 0.04 |
| Hyperlipidemia | - | 2.21 ± 0.25 Δ | 1.23 ± 0.11 Δ | 0.70 ± 0.04 Δ | 0.82 ± 0.13 Δ |
| U | 250 | 1.34 ± 0.35 * | 0.85 ± 0.05 | 0.41 ± 0.01 * | 0.81 ± 0.05 |
| HU (low dose) | 125 | 1.34 ± 0.06 * | 0.79 ± 0.02 | 0.41 ± 0.02 * | 0.88 ± 0.02 |
| HU(middle dose) | 250 | 1.30 ± 0.10 ** | 0.72 ± 0.14 * | 0.43 ± 0.05 | 0.93 ± 0.13 |
| HU (high dose) | 500 | 1.39 ± 0.18 | 0.78 ± 0.06 | 0.43 ± 0.04 | 0.99 ± 0.01 * |
| Positive control | 500 | 1.77 ± 0.18 | 0.91 ± 0.22 | 0.50 ± 0.06 | 1.11 ± 0.07 ** |
| Female | |||||
| Normal control | - | 1.89 ± 0.13 | 0.72 ± 0.03 | 0.41 ± 0.03 | 1.20 ± 0.09 |
| Hyperlipidemia | - | 2.25 ± 0.47 Δ | 1.21 ± 0.09 Δ | 0.60 ± 0.05 Δ | 0.84 ± 0.25 Δ |
| U | 250 | 1.63 ± 0.30 * | 0.76 ± 0.08 | 0.38 ± 0.06 | 0.98 ± 0.14 |
| HU (low dose) | 125 | 1.26 ± 0.04 **,# | 0.65 ± 0.04 * | 0.30 ± 0.01 ** | 1.05 ± 0.07 * |
| HU(middle dose) | 250 | 1.26 ± 0.04 **,# | 0.67 ± 0.05 * | 0.32 ± 0.01 | 0.91 ± 0.07 |
| HU (high dose) | 500 | 1.42 ± 0.11 | 0.67 ± 0.07 * | 0.31 ± 0.05 * | 0.91 ± 0.09 |
| Positive control | 500 | 1.75 ± 0.17 | 0.81 ± 0.16 | 0.38 ± 0.02 | 1.15 ± 0.12 ** |


2.1.4. LXR mRNA Expression


2.1.5. PPARγ mRNA Expression


2.2. Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of Natural Polysaccharide Ulvan
3.3. Preparation of High Sulfate Content Ulvan HU
3.4. Chemical Analysis
3.5. Animals and Experimental Design
3.5.1. Animals
3.5.2. Determination of TC, TG, LDL-C and HDL-C Levels
3.5.3. RT-PCR Analysis
| Gene | Forward Primers | Reverse Primers |
|---|---|---|
| FXR | 5′-AGCCCGAGAACCCTCAGCATT-3′ | 5′-TCATTCACCCTCCAAGACATCAGC-3′ |
| LXR | 5′-GAACGAGCTATGCAGTGTATGTGGG-3′ | 5′-GCTCCTCTTCTTGACGCTTCAGTTT-3′ |
| PPARγ | 5′-CAAGGAGGCAGAGGTCCGATTC-3′ | 5′-CTTGGGTTCCATGATGTCGCAG-3′ |
| β actin | 5′-GAACCCTAAGGCCAACCGTGAA-3′ | 5′-CGACCAGAGGCATACAGGGACA-3′ |
3.6. Statistical Analysis
Acknowledgments
Author Contributions
Abbreviations
| U | ulvan |
| HU | high sulfate content ulvan |
| SO3–DMF | sulfur trioxide/N,N-dimethylformamide |
| TC | total cholesterol |
| TG | triglyceride |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| FXR | farnesoid X receptor |
| LXR | liver X receptor |
| PPAR | peroxisome proliferator-activated receptor |
Conflicts of Interest
References
- Ballantyne, C.M. Low-density lipoproteins and risk for coronary artery disease. Am. J. Cardiol. 1998, 82, 3–12. [Google Scholar] [CrossRef]
- Tzang, B.S.; Yang, S.F.; Fu, S.G.; Yang, H.C.; Sun, H.L.; Chen, Y.C. Effects of flaxseed oil on cholesterol metabolism of hamsters. Food Chem. 2009, 114, 1450–1455. [Google Scholar] [CrossRef]
- Graham, D.J.; Staffa, J.A.; Shatin, D.; Andrade, S.E.; Schech, S.D.; Grenade, L.; Gurwitz, J.H.; Chan, K.A.; Goodman, M.J.; Platt, R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004, 292, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.J.; Jeong, S.J.; Lee, M.H.; Kim, S.H. Essential oil of Pinus koraiensis leaves exerts antihyperlipidemic effects via up-regulation of low-density lipoprotein receptor and inhibition of acyl-coenzyme A: Cholesterol acyltransferase. Phytother. Res. 2012, 26, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.R.; Nabipour, I. The hypolipidemic effect of Otrullus colocynthis on patients with hyperlipidemia. Pak. J. Biol. Sci. 2010, 13, 1202–1207. [Google Scholar] [PubMed]
- Bavarva, J.H.; Narasimhacharya, A.V. Antihyperglycemic and hypolipidemic effects of Costus speciosus in alloxan induced diabetic rats. Phytother. Res. 2008, 22, 620–626. [Google Scholar] [PubMed]
- Liao, F.H.; Shieh, M.J.; Chang, N.C.; Chien, Y.W. Chitosan supplementation lowers serum lipids and maintains normal calcium, magnesium, and iron status in hyperlipidemic patientsa. Nutr. Res. 2007, 27, 146–151. [Google Scholar] [CrossRef]
- Zhang, H.L.; Tao, Y.; Guo, J.; hu, Y.M.; Su, Z.Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Huang, L.Y.; Liu, X.L.; Liu, D.M.; Zhang, Q.B.; Liu, S.M. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Lahaye, M.; Jegon, D. Chemical and physical-chemical characteristics of dietary fibers from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J. Appl. Phycol. 1993, 5, 195–200. [Google Scholar] [CrossRef]
- Guan, H.; Wang, S.G. The Chinese Marine Materia Medica; Press of Shanghai Science Technology: Shanghai, China, 2009; p. 292. [Google Scholar]
- Qi, H.M.; Zhang, Q.B.; Zhao, T.T.; Hu, R.G.; Zhang, K.; Li, Z.E. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg. Med. Chem. Lett. 2006, 16, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Z.; Zhang, Q.B.; Li, N.; Xu, Z.H.; Wang, Y.M.; Li, Z.E. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar]
- Wang, K.; Qi, H.M.; Li, Y.L.; Xu, H.T.; Liu, S.M. The study of Ulva pertusa and its derivative on anti-tumor activity in vitro. Chin. J. Mar. Drugs 2014, 33, 19–23. [Google Scholar]
- Luo, Z.Y.; Chen, Y.Y.; Wang, C.; Qu, M.Y.; Zhang, A.W.Z.; Xiong, S. Extraction and purification of anti-viral proteoglycan from Ulva Pertusa and its activity aganinst coxsackie virus B3. Lishizhen Med. Mater. Med. Res. 2010, 21, 1090–1093. [Google Scholar]
- Thongngam, M.; McClements, D.J. Isothermal titration calorimetry study of the interactions between chitosan and a bile salt (sodium taurocholate). Food Hydrocolloids 2005, 19, 813–819. [Google Scholar] [CrossRef]
- Fan, S.J.; Guo, L.; Zhang, Y.; Sun, Q.H.; Yang, B.C.; Huang, C. Okra polysaccharide improves metabolic disorders in high-fat diet-induced obese C57BL/6 mice. Mol. Nutr. Food Res. 2013, 57, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Ma, X.J.; Ye, M.; Yuan, R.Y.; Wu, Y.N. Purification, structure, lipid lowering and liver protecting effects of polysaccharide from Lachnum YM281. Carbohydr. Polym. 2013, 98, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Goode, E.; Chen, J.; Oro, A.E.; Bradley, D.J.; Perlmann, T.; Noonan, D.J.; Burka, L.T.; McMorris, T.; Lamph, W.W.; et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995, 81, 687–693. [Google Scholar] [CrossRef]
- Lu, T.T.; Repa, J.J.; Mangelsdorf, D.J. Oraphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J. Biol. Chem. 2001, 276, 37735–37738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kast-Woelbern, H.R.; Edwards, P.A. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J. Biol. Chem. 2003, 278, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Edwards, P.A. FXR signaling in metabolic disease. FEBS. Lett. 2008, 582, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Maloney, P.R.; Parks, D.J.; Haffner, C.D.; Fivush, A.M.; Chandra, G.; Plunket, K.D.; Creech, K.L.; Moore, L.B.; Wilson, J.G.; Lewis, M.C.; et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 2000, 43, 2971–2974. [Google Scholar] [CrossRef] [PubMed]
- Kast, H.R.; Nguyen, C.M.; Sinal, C.J.; Jones, S.A.; Laffitte, B.A.; Reue, K.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: A molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 2001, 15, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Castellani, L.W.; Sinal, C.J.; Conzalez, F.J.; Edwards, P.A. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004, 18, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995, 9, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Spyridon, M.; Moraes, L.; Jones, C.I.; Sage, T.; Sasikumar, P.; Bucci, G.; Gibbins, J.M. LXR as a novel antithrombotic target. Blood 2011, 117, 5751–5761. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Dai, X.Y.; Chen, Q.; Zang, J.N.; Deng, L.L.; Liu, Y.H.; Ying, H.Z. Hypolipidemic and antioxidant activities of polysaccharides from Rosaw Laevigatae Fructus in rats. Carbohydr. Polym. 2013, 5, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Peng, G.; Li, Q.; Wen, S.; Huang, T.H.; Roufogalis, B.D.; Yamahara, J. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese Zucker rats. Life Sci. 2004, 75, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.R.S.; Feitosa, J.P.A.; Freitas, A.L.P.; Paula, R.C.M.de. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491–498. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.D.; Li, N.; Zhang, Q.B.; Zhang, H. The profective effect of fucoidan in rats with streptozotocin-induced diabetic nephropathy. Mar. Drugs 2014, 12, 3292–3306. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mediavilla, V.; Villares, C.; Culebras, J.M.; Bayon, J.E.; Gonzalez-Gallego, J. Effects of dietary β-cyclodextrin in hypercholesterolaemic rats. Pharmacol. Toxicol. 2003, 92, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustiq, K.D.; Mangelsdorf, D.F.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kawada, T.; Coto, T.; Yamamoto, T.; Taimatsu, A.; Matsui, N.; Kimura, K.; Saito, M.; Hosokawa, M.; Miyashita, K.; et al. Dual action of isoprenols from herbal medicines on both PPARγ and PPARα in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002, 514, 315–322. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Sheng, J. The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats. Mar. Drugs 2015, 13, 3407-3421. https://doi.org/10.3390/md13063407
Qi H, Sheng J. The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats. Marine Drugs. 2015; 13(6):3407-3421. https://doi.org/10.3390/md13063407
Chicago/Turabian StyleQi, Huimin, and Jiwen Sheng. 2015. "The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats" Marine Drugs 13, no. 6: 3407-3421. https://doi.org/10.3390/md13063407
APA StyleQi, H., & Sheng, J. (2015). The Antihyperlipidemic Mechanism of High Sulfate Content Ulvan in Rats. Marine Drugs, 13(6), 3407-3421. https://doi.org/10.3390/md13063407