Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products
Abstract
:1. Introduction
2. Results and Discussion
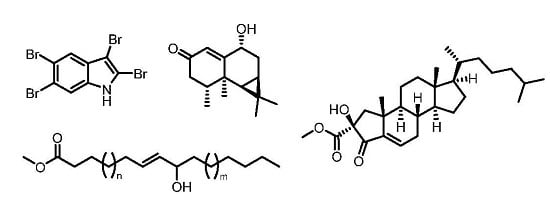
2.1. Laurencia complanata

| Microbes | Zone of inhibition (Ø in mm) b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laurencia complanata | Grateloupia sp. | Gracilaria corticata | Halymenia sp. | Spyridia sp. | Meta-mastophora sp. | Calloseris sp. | Neurymenia fraxinifolia | ||||
| Crude extract | Mixture of 1 and 2 | Compound 3 | MeOH fraction (4) | ||||||||
| Enterobacter cloacae ATCC 700323 | 6.5 | nt | nt | nt | 6 | 6.5 | 6 | 6.5 | 6 | 6 | 6.5 |
| Klebsiella oxytoca ATCC 8724 | 7 | nt | nt | nt | 6 | 6.5 | 6 | 6.5 | 6 | 6 | 6.5 |
| Escherichia coli | 7 | nt | nt | nt | 6 | 6.5 | 6 | 6 | 6 | 6 | 6.5 |
| Salmonella enteridis | 6 | nt | nt | nt | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Bacillus cereus ATCC 13061 | 19.5 | 6.5 | 11 | 7.5 | 7 | 6.5 | 8 | 6.5 | 6.5 | 6 | 6.5 |
| Staphylococcus aureus ATCC 11632 | 20 | 6 | 12 | 6 | 6.5 | 8 | 9 | 6.5 | 6 | 6 | 6.5 |
| Streptococcus pneumoniae ATCC 6301 | 20 | 6.5 | 12 | 6 | 6 | 6 | 12 | 6 | 8 | 6 | 6 |
| Candida albicans | 11.5 | 6.5 | 8.5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
2.2. Grateloupia sp.
2.3. Gracilaria corticata (Gracilariaceae)

2.4. Halymenia sp. (Halymeniaceae)
2.5. Spyridia sp. (Spyridiaceae)

2.6. Metamastophora sp. (Corallinaceae)
2.7. Calloseris sp. (Delesseriaceae)

| Position | 9 from Calloseris sp. δC (150 MHz, CDCl3) | 9 from Dendronephthya sp. [26] δC (125 MHz, CDCl3) | 10 from Calloseris sp. δC (150 MHz, CDCl3) | 10 from Phorbas amaranthus [27] δC (100 MHz, CDCl3) |
|---|---|---|---|---|
| 1 | 46.67 | 46.7 | 46.66 | nr |
| 2 | 79.79 | 79.8 | 79.88 | 79.9 |
| 3 | 172.90 | 172.9 | 173.35 | 173.4 |
| 4 | 201.05 | 201.0 | 200.89 | 200.9 |
| 5 | 145.17 | 145.2 | 145.09 | 145.1 |
| 6 | 136.00 | 135.9 | 136.26 | 136.3 |
| 7 | 32.17 | 32.2 | 32.19 | nr |
| 8 | 32.32 | 32.4 | 32.31 | nr |
| 9 | 49.65 | 49.7 | 49.64 | nr |
| 10 | 39.89 | 39.9 | 39.91 | nr |
| 11 | 21.78 | 21.8 | 21.77 | nr |
| 12 | 39.34 | 39.5 | 39.33 | nr |
| 13 | 42.90 | 42.9 | 42.89 | nr |
| 14 | 56.25 | 56.1 | 56.25 | nr |
| 15 | 24.32 | 24.3 | 24.32 | nr |
| 16 | 28.12 | 28.1 | 28.12 | nr |
| 17 | 56.09 | 56.3 | 56.07 | nr |
| 18 | 12.01 | 12.0 | 12.00 | nr |
| 19 | 22.08 | 22.1 | 22.07 | nr |
| 20 | 35.73 | 35.7 | 35.72 | 35.7 |
| 21 | 18.73 | 18.7 | 18.72 | 18.7 |
| 22 | 36.15 | 36.2 | 36.14 | 36.1 |
| 23 | 23.83 | 23.9 | 23.83 | 23.8 |
| 24 | 39.47 | 39.3 | 39.47 | 39.5 |
| 25 | 28.00 | 28.0 | 28.00 | 28.0 |
| 26 | 22.81 | 22.8 | 22.54 | 22.5 |
| 27 | 22.55 | 22.5 | 22.80 | 22.8 |
| 1′ | 62.66 | 62.7 | 53.37 | 53.4 |
| 2′ | 14.08 | 14.2 | − | − |
2.8. Neurymenia fraxinifolia (Rhodomelaceae)

3. Experimental Section
3.1. Plant Material
3.2. Extraction and Isolation
3.3. Spectroscopic Characterization
3.4. Biological Testing
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflict of Interest
References
- Jha, R.K.; Zi-rong, X. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–49. [Google Scholar] [PubMed]
- De Almeida, C.L.F.; de S. Falcão, H.; de M. Lima, G.R.; de A. Montenegro, C.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza, M.d.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef] [PubMed]
- Mollion, J. On the agar from Gelidium madagascariense (Rhodophyceae). Hydrobiologia 1992, 232, 43–46. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; France, D.; Cornell-Kennon, S.; Gerwick, W.H. DNA methyl transferase inhibiting halogenated monoterpenes from the Madagascar red marine alga Portieria hornemannii. J. Nat. Prod. 2006, 69, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Rahelivao, M.P.; Andriamanantoanina, H.; Heyraud, A.; Rinaudo, M. Structure and rheological behaviour of agar extracted from Madagascar sea coast algae. Open Macromol. J. 2014, 7, 1–6. [Google Scholar] [CrossRef]
- Carter, G.T.; Rinehart, K.L., Jr.; Li, L.H.; Kuentzel, S.L.; Connor, J.L. Brominated indoles from Laurencia brongniartii. Tetrahedron Lett. 1978, 19, 4479–4482. [Google Scholar] [CrossRef]
- Suárez-Castillo, O.R.; Beiza-Granados, L.; Meléndez-Rodríguez, M.; Álvarez-Hernández, A.; Morales-Ríos, M.S.; Joseph-Nathan, P. Synthesis of bromoindole alkaloids from Laurencia brongniartii. J. Nat. Prod. 2006, 69, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Occurrence of halogenated alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; Volume 71, pp. 1–165. [Google Scholar]
- Tanaka, J.; Higa, T.; Bernardinelli, G.; Jefford, C.W. Sulfur-containing polybromoindoles from the red alga Laurencia brongniartii. Tetrahedron 1989, 45, 7301–7310. [Google Scholar] [CrossRef]
- Rao, C.B.; Satyanarayana, C.; Rao, D.V.; Fahy, E.; Faulkner, D.J. Metabolites of Aplysia dactylomela from the Indian ocean. Indian J. Chem. B 1989, 28B, 322–325. [Google Scholar]
- Ji, N.-Y.; Li, X.-M.; Ding, L.-P.; Wang, B.-G. Aristolane sesquiterpenes and highly brominated indoles from the marine red alga Laurencia similis (Rhodomelaceae). Helv. Chim. Acta 2007, 90, 385–391. [Google Scholar] [CrossRef]
- Ji, N.-Y.; Li, X.-M.; Cui, C.-M.; Wang, B.-G. Terpenes and polybromoindoles from the marine red alga Laurencia decumbens (Rhodomelaceae). Helv. Chim. Acta 2007, 90, 1731–1736. [Google Scholar] [CrossRef]
- Itokawa, H.; Masuyama, K.; Morita, H.; Takeya, K. Cytotoxic sesquiterpenes from Nardostachys chinensis. Chem. Pharm. Bull. 1993, 41, 1183–1184. [Google Scholar] [CrossRef] [PubMed]
- Křepinský, J.; Jommi, G.; Samek, Z.; Šorm, F. The sesquiterpenic constituents of Aristolochia debilis Sieb. et Zucc. The structure of debilone. Collect. Czech. Chem. Commun. 1970, 35, 745–748. [Google Scholar] [CrossRef]
- Rücker, G. 9-Hydroxy-Δ1(10)-aristolenon-(2) (Debilon) aus Nardostachys chinensis. Planta Med. 1970, 18, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Zhang, L.; Zheng, Q.-T.; Xu, L.-Z.; Yang, S.-L. Terpenoids from the roots and rhizomes of Nardostachys chinensis. J. Nat. Prod. 2005, 68, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Han, X.H.; Lee, C.; Lee, D.; Lee, C.-K.; Hong, J.T.; Kim, Y.; Lee, M.K.; Hwang, B.Y. Inhibitory constituents of Nardostachys chinensis on nitric oxide production in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2012, 22, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.W. The distribution of sterols in algae. Lipids 1971, 6, 120–127. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Kato, M. Triterpenes and steroids in the gel of Aloe vera (L.) Burm. f. Tokyo Kasei Daigaku Kenkyu Kiyo 1998, 38, 157–160. [Google Scholar]
- Abd El-Aty, A.M.; Kim, I.-K.; Kim, M.-R.; Lee, C.; Shim, J.-H. Determination of volatile organic compounds generated from fresh, white and red Panax ginseng (c. A. Meyer) using a direct sample injection technique. Biomed. Chromatogr. 2008, 22, 556–562. [Google Scholar]
- Chang, S.-H.; Bae, J.-H.; Hong, D.-P.; Choi, K.-D.; Kim, S.-C.; Her, E.; Kim, S.-H.; Kang, C.-D. Dryopteris crassirhizoma has anti-cancer effects through both extrinsic and intrinsic apoptotic pathways and G0/G1 phase arrest in human prostate cancer cells. J. Ethnopharmacol. 2010, 130, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vujić, Đ.N.; Ačanski, M.M.; Bodroža-Solarov, M.I.; Hristov, N.S.; Krunić, M.N. Performance of GC-MS analysis for differentiation of various types of flour by creating dendrogram of liposoluble extract. Chem. Ind. Chem. Eng. Q. 2012, 18, 555–561. [Google Scholar] [CrossRef]
- Sanubol, A.; Chaveerach, A.; Sudmoon, R.; Tanee, T.; Noikotr, K. Betel-like-scented piper plants as diverse sources of industrial and medicinal aromatic chemicals. Chiang Mai J. Sci. 2014, 41, 1171–1181. [Google Scholar]
- Li, G.; Deng, Z.; Guan, H.; van Ofwegen, L.; Proksch, P.; Lin, W. Steroids from the soft coral Dendronephthya sp. Steroids 2005, 70, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Masuno, M.N.; Pawlik, J.R.; Molinski, T.F. Phorbasterones A−D, cytotoxic nor-ring A steroids from the sponge Phorbas amaranthus. J. Nat. Prod. 2004, 67, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Tischler, M.; Ayer, S.W.; Andersen, R.J.; Mitchell, J.F.; Clardy, J. Anthosterones A and B, ring A contracted steroids from the sponge Anthoracuata graceae. Can. J. Chem. 1988, 66, 1173–1178. [Google Scholar] [CrossRef]
- Migliuolo, A.; Piccialli, V.; Sica, D. Steroidal ketones from the sponge Geodia cydonium. J. Nat. Prod. 1990, 53, 1262–1266. [Google Scholar] [CrossRef]
- Fieser, L.F. Δ4-Cholesten-3,6-dione. Org. Synth. 1955, 35, 36. [Google Scholar]
- Dong, J.; Chen, W.; Wang, S.; Zhang, J.; Li, H.; Guo, H.; Man, Y.; Chen, B. Jones oxidation and high performance liquid chromatographic analysis of cholesterol in biological samples. J. Chromatogr. B 2007, 858, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Garwood, R.F.; Khambay, B.P.S.; Weedon, B.C.L.; Frankel, E.N. Allylic hydroperoxides from the autoxidation of methyl oleate. J. Chem. Soc. Chem. Commun. 1977, 364–365. [Google Scholar] [CrossRef]
- Frankel, E.N.; Garwood, R.F.; Khambay, B.P.S.; Moss, G.P.; Weedon, B.C.L. Stereochemistry of olefin and fatty acid oxidation. Part 3. The allylic hydroperoxides from the autoxidation of methyl oleate. J. Chem. Soc. Perkin Trans. 1 1984, 2233–2240. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Pizzale, L.; Lercker, G. Chromatographic determination of position and configuration isomers of methyl oleate hydroxides from corresponding hydroperoxides. Chromatographia 1993, 36, 61–64. [Google Scholar] [CrossRef]
- Minami, Y.; Yokoyama, K.; Bando, N.; Kawai, Y.; Terao, J. Occurrence of singlet oxygen oxygenation of oleic acid and linoleic acid in the skin of live mice. Free Radic. Res. 2008, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Mills, K.A.; Carter, R.L. A mechanistic study of oleate autoxidation: Competing peroxyl H-atom abstraction and rearrangement. J. Am. Chem. Soc. 1994, 116, 6690–6696. [Google Scholar] [CrossRef]
- Koshino, H.; Togiya, S.; Yoshihara, T.; Sakamura, S.; Shimanuki, T.; Sato, T.; Tajimi, A. Four fungitoxic C-18 hydroxy unsaturated fatty acids from stromata of Epichloe typhina. Tetrahedron Lett. 1987, 28, 73–76. [Google Scholar] [CrossRef]
- Vig, O.P.; Bari, S.S.; Sethi, M.K.; Sattar, M.A. Synthesis of ethyl (±)-10-hydroxy-8(E)-octadecenoate and ethyl (±)-9-hydroxy-10(E)-octadecenoate. Indian J. Chem. B 1991, 30B, 608–610. [Google Scholar] [CrossRef]
- Adam, W.; Andler, S.; Saha-Möller, C.R. DNA cleavage induced by oxyl radicals generated in the photosensitized decomposition of fatty ester hydroperoxides derived from oleic and linoleic acid. Arch. Biochem. Biophys. 1998, 349, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Andler, S.; Saha-Möller, C.R.; Schönberger, A. Inhibitory effect of ethyl oleate hydroperoxide and alcohol in photosensitized oxidative DNA damage. J. Photochem. Photobiol. B Biol. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Liu, Y.; Gribble, G.W. Syntheses of polybrominated indoles from the red alga Laurencia brongniartii and the brittle star Ophiocoma erinaceus. J. Nat. Prod. 2002, 65, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.B.; Ghellamallah, C.; Male, L.; Spencer, N.; Grainger, R.S. Regioselective dibromination of methyl indole-3-carboxylate and application in the synthesis of 5,6-dibromoindoles. Org. Biomol. Chem. 2011, 9, 5021–5023. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, M.; Hashimoto, T.; Noma, Y.; Asakawa, Y. Biotransformation of aristolane- and 2,3-secoaromadendrane-type sesquiterpenoids having a 1,1-dimethylcyclopropane ring by Chlorella fusca var. vacuolata, Mucor species, and Aspergillus niger. Chem. Pharm. Bull. 2006, 54, 861–868. [Google Scholar] [CrossRef]
- Rücker, G.; Kretzschmar, U. Darstellung von 1.8.9.10-tetradehydro-aristolanon-(2) und 9-hydroxy-1(10)-aristolenon-(2) (Debilon). Justus Liebigs Ann. Chem. 1971, 748, 211–213. [Google Scholar] [CrossRef]
- Corbett, Y.; Herrera, L.; Gonzalez, J.; Cubilla, L.; Capson, T.L.; Coley, P.D.; Kursar, T.A.; Romero, L.I.; Ortega-Barria, E. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am. J. Trop. Med. Hyg. 2004, 70, 119–124. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.-J. Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Mar. Drugs 2015, 13, 4197-4216. https://doi.org/10.3390/md13074197
Rahelivao MP, Gruner M, Andriamanantoanina H, Andriamihaja B, Bauer I, Knölker H-J. Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Marine Drugs. 2015; 13(7):4197-4216. https://doi.org/10.3390/md13074197
Chicago/Turabian StyleRahelivao, Marie Pascaline, Margit Gruner, Hanta Andriamanantoanina, Bakolinirina Andriamihaja, Ingmar Bauer, and Hans-Joachim Knölker. 2015. "Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products" Marine Drugs 13, no. 7: 4197-4216. https://doi.org/10.3390/md13074197
APA StyleRahelivao, M. P., Gruner, M., Andriamanantoanina, H., Andriamihaja, B., Bauer, I., & Knölker, H. -J. (2015). Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Marine Drugs, 13(7), 4197-4216. https://doi.org/10.3390/md13074197