Structure-Activity Relationships of the Bioactive Thiazinoquinone Marine Natural Products Thiaplidiaquinones A and B
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

2.2. Biology
2.2.1. Inhibition of ROS Generation

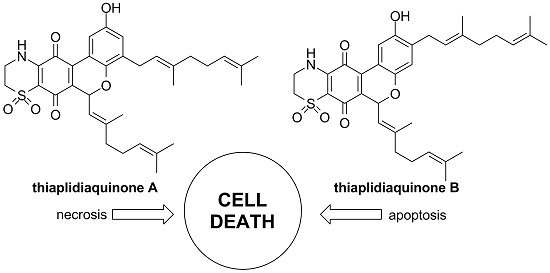
2.2.2. Apoptosis vs. Necrosis in Jurkat Cells

2.2.3. In Vitro Cytotoxicity Screening at the NCI
2.2.4. Antimalarial and Anti-Farnesyltransferase Activities
| Compound | P. falc. a | FTase (T. b.) b | FTase (H) c |
|---|---|---|---|
| 1 | >17 d | 0.74 ± 0.20 | 0.78 ± 0.17 |
| 2 | >17 d | 3.04 ± 0.30 | 1.22± 0.068 |
| 3 | 4.56 ± 0.76 d | 0.22 ± 0.034 | 0.14 ± 0.0017 |
| 4 | 4.39 ± 0.77 d | 0.098 ± 0.008 | 0.054 ± 0.005 |
| 5 | 2.2 e | 3.90 ± 0.60 | 3.70 ± 0.60 |
| 6 | 2.3 e | 6.16 ± 1.40 | 1.64 ± 0.30 |
| Chloroquine f | 0.45 d, 0.0063 e | ||
| FTI 276 f | 0.010 ± 0.002 | 0.015 ± 0.004 |
3. Experimental Section
3.1. Chemistry
3.2. Biology
3.2.1. Cell Culture
3.2.2. Intracellular ROS
3.2.3. Jurkat Cell Cytotoxicity Assay
3.2.4. NCI Evaluation
3.2.5. Antimalarial and Anti-Farnesyltransferase Evaluation
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zubía, E.; Ortega, M.J.; Salvá, J. Natural products chemistry in marine ascidians of the genus Aplidium. Mini Rev. Org. Chem. 2005, 2, 389–399. [Google Scholar]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar]
- Chan, S.T.S.; Pearce, A.N.; Januario, A.H.; Page, M.J.; Kaiser, M.; McLaughlin, R.J.; Harper, J.L.; Webb, V.L.; Barker, D.; Copp, B.R. Anti-inflammatory and antimalarial meroterpenoids from the New Zealand ascidian Aplidium scabellum. J. Org. Chem. 2011, 76, 9151–9156. [Google Scholar]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Macho, A.; Menna, M.; Muñoz, E. Antitumor effects of two novel naturally occurring terpene quinones isolated from the Mediterranean ascidian Aplidium conicum. J. Med. Chem. 2005, 48, 3410–3416. [Google Scholar]
- Khalil, I.M.; Barker, D.; Copp, B.R. Biomimetic synthesis of thiaplidiaquinones A and B. J. Nat. Prod. 2012, 75, 2256–2260. [Google Scholar]
- Carbone, A.; Lucas, C.L.; Moody, C.J. Biomimetic synthesis of the apoptosis-inducing thiazinoquinone thiaplidiaquinone A. J. Org. Chem. 2012, 77, 9179–9189. [Google Scholar]
- Emmendorffer, A.; Hecht, M.; Lohmann-Matthes, M.-L.; Roesler, J. A fast and easy method to determine the production of reactive oxygen intermediates by human and murine phagocytes using dihydrorhodamine 123. J. Immunol. Methods 1990, 131, 269–275. [Google Scholar]
- Villena, J.; Henriquez, M.; Torres, V.; Moraga, F.; Diaz-Elizondo, J.; Arredondo, C.; Chiong, M.; Olea-Azar, C.; Stutzin, A.; Lavandero, S.; et al. Ceramide-induced formation of ROS and ATP depletion trigger necrosis in lymphoid cells. Free Radic. Biol. Med. 2008, 44, 1146–1160. [Google Scholar]
- Maschke, M.; Alborzinia, H.; Lieb, M.; Wolfl, S.; Metzler-Nolte, N. Structure-activity relationship of trifluoromethyl-containing metallocenes: Electrochemistry, lipophilicity, cytotoxicity, and ROS production. ChemMedChem 2014, 9, 1188–1194. [Google Scholar] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [PubMed]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Menna, M.; Calzado, M.A.; Muñoz, E.; Bonadies, F.; Guiso, M.; Sanasi, M.F.; Cocco, G.; et al. Synthesis of structurally simplified analogues of aplidinone A, a pro-apoptotic marine thiazinoquinone. Bioorg. Med. Chem. 2010, 18, 719–727. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Longeon, A.; Copp, B.R.; Roué, M.; Dubois, J.; Valentin, A.; Petek, S.; Debitus, C.; Bourguet-Kondracki, M.-L. New bioactive halenaquinone derivatives from South Pacific marine sponges of the genus Xestospongia. Bioorg. Med. Chem. 2010, 18, 6006–6011. [Google Scholar] [CrossRef] [PubMed]
- Ménan, H.; Banzouzi, J.-T.; Hocquette, A.; Pélissier, Y.; Blache, Y.; Koné, M.; Mallié, M.; Assi, L.A.; Valentin, A. Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J. Ethnopharmacol. 2006, 105, 131–136. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, J.L.; Khalil, I.M.; Shaw, L.; Bourguet-Kondracki, M.-L.; Dubois, J.; Valentin, A.; Barker, D.; Copp, B.R. Structure-Activity Relationships of the Bioactive Thiazinoquinone Marine Natural Products Thiaplidiaquinones A and B. Mar. Drugs 2015, 13, 5102-5110. https://doi.org/10.3390/md13085102
Harper JL, Khalil IM, Shaw L, Bourguet-Kondracki M-L, Dubois J, Valentin A, Barker D, Copp BR. Structure-Activity Relationships of the Bioactive Thiazinoquinone Marine Natural Products Thiaplidiaquinones A and B. Marine Drugs. 2015; 13(8):5102-5110. https://doi.org/10.3390/md13085102
Chicago/Turabian StyleHarper, Jacquie L., Iman M. Khalil, Lisa Shaw, Marie-Lise Bourguet-Kondracki, Joëlle Dubois, Alexis Valentin, David Barker, and Brent R. Copp. 2015. "Structure-Activity Relationships of the Bioactive Thiazinoquinone Marine Natural Products Thiaplidiaquinones A and B" Marine Drugs 13, no. 8: 5102-5110. https://doi.org/10.3390/md13085102
APA StyleHarper, J. L., Khalil, I. M., Shaw, L., Bourguet-Kondracki, M. -L., Dubois, J., Valentin, A., Barker, D., & Copp, B. R. (2015). Structure-Activity Relationships of the Bioactive Thiazinoquinone Marine Natural Products Thiaplidiaquinones A and B. Marine Drugs, 13(8), 5102-5110. https://doi.org/10.3390/md13085102