Determination of FVIIa-sTF Inhibitors in Toxic Microcystis Cyanobacteria by LC-MS Technique
Abstract
:1. Introduction
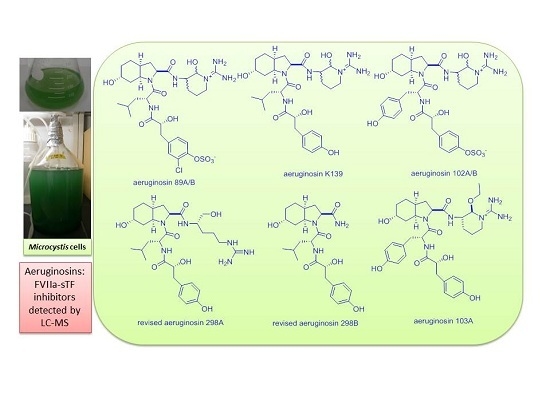
2. Results and Discussion
| Structural Class | Compound Name | Serine Protease Inhibitory Assays (µg/mL) | |
|---|---|---|---|
| 1. Aeruginopeptins | 95-A (1) 95-B (2) | thrombin, 10, 1: | (−) |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| 228-B (3) | thrombin, 10, 1: | (−) | |
| fVIIa, 100, 10: | (+) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| 917S-A (4) | thrombin, 10: | (−) | |
| 917S-B (5) | fVIIa, 100, 10: | (−) | |
| 917S-C (6) | fVIIa-sTF, 10, 1: | (−) | |
| 2. Anabaenopeptins | A (7) | thrombin, 10: | (−) |
| B (8) | fVIIa, 100, 10: | (−) | |
| C (9) | fVIIa-sTF, 10, 1: | (−) | |
| oscillapeptin G (10) oscillapeptilide 97A (11) | thrombin, 10, 1: | (−) | |
| fVIIa, 100, 10: | (+) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| oscillapeptilide 97B (12) | thrombin, 10, 1: | (−) | |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| 3. Anabaenapeptilides | 90-A (13) 90-B (14) | thrombin, 10, 1: | (−) |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| 202-A (15) 202-B (16) | thrombin, 10:1: | (−) | |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| oscillamide Y (17) | thrombin, 10, 1: | (−) | |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 10, 1: | (−) | ||
| 4. Microcystins | LR (18) | fVIIa-sTF, 100, 10, 1: | (−) |
| RR (19) | fVIIa-sTF, 100, 10, 1: | (−) | |
| YR (20) | fVIIa-sTF, 100, 10, 1: | (−) | |
| 5. Spumigins | A (21) | thrombin: 100, 10, 1: | (+,−,−) |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| J (22) | thrombin: 10, 1: | (+) | |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 100, 10, 1: | (−) | ||
| 6. Other Peptides | nostophycin (23) | thrombin, 10: | (−) |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 10, 1: | (−) | ||
| microcyclamide (24) microviridin (25) | thrombin, 10, 1: | (−) | |
| fVIIa, 100, 10: | (−) | ||
| fVIIa-sTF, 10, 1: | (−) | ||

| No. | M. aeruginosa Strain | % MeOH Fraction | Retention Time,tR (min) | (m/z) | Ions Detected | Compounds Detected |
|---|---|---|---|---|---|---|
| 1 | NIES-89 | 40 | 8.3 | 637.89 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) |
| 8.3 | 717.60 | [M + H]+ | (26/27) | |||
| 8.8 | 638.05 | [M − SO3 + H]+ | (26/27) | |||
| 8.8 | 717.63 | [M + H]+ | (26/27) | |||
| 9.8 | 638.07 | [M − SO3 + H]+ | (26/27) | |||
| 10.2 | 638.03 | [M − SO3 + H]+ | (26/27) | |||
| 45 | 8.3 | 637.81 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) | ||
| 8.3 | 717.68 | [M + H]+ | (26/27) | |||
| 9.9 | 637.91 | [M − SO3 + H]+ | (26/27) | |||
| 9.9 | 717.40 | [M + H]+ | (26/27) | |||
| 18.9 | 996.05 | [M + H]+ | microcystin-LR (18) | |||
| 2 | K139 | 60 | 5.1 | 603.50 | [M + H]+ | aeruginosin K139 (30) |
| 5.1 | 621.39 | [M + H2O + H]+ | (30) | |||
| 6.2 | 603.34 | [M + H]+ | (30) | |||
| 7.6 | 603.33 | [M + H]+ | (30) | |||
| 8.4 | 603.34 | [M + H]+ | (30) | |||
| 3 | M228 | 60 | 5.2 | 605.48 | [M + H]+ | aeruginosin 298A (32) |
| 6.3 | 621.34 | [M + H2O + H]+ | aeruginosin K139 (30) | |||
| 7.7 | 603.41 | [M + H]+ | (30) | |||
| 8.5 | 603.35 | [M + H]+ | (30) | |||
| 14.9 | 1031.91 | [M + H − H2O]+ | aeruginopeptin 228-B (3) | |||
| 14.9 | 1049.43 | [M + H]+ | (3) | |||
| 15.5 | 1028.27 | [M + H − H2O]+ | aeruginopeptin 228-A (33) | |||
| 18.4 | 1046.05 | [M + H]+ | microcystin-YR (20) | |||
| 4 | TAC 95 | 40 | 5.5 | 605.65 | [M + H]+ | aeruginosin 298A (32) |
| 5.5 | 621.61 | [M + H2O + H]+ | aeruginosin K139 (30) | |||
| 6.5 | 603.61 | [M + H]+ | (30) | |||
| 7.8 | 603.78 | [M + H]+ | (30) | |||
| 8.5 | 603.77 | [M + H]+ | (30) | |||
| 60 | 7.7 | 603.62 | [M + H]+ | aeruginosin K139 (30) | ||
| 8.5 | 603.59 | [M + H]+ | (30) | |||
| 8.8 | 603.92 | [M + H]+ | (30) | |||
| 15.1 | 1132.92 | [M + H − H2O]+ | aeruginopeptin 95B (2) | |||
| 15.4 | 1129.04 | [M + H − H2O]+ | aeruginopeptin 95A (1) | |||
| 5 | NIES-102 | 40 | 1.8 | 653.86 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) |
| 1.8 | 733.71 | [M + H]+ | (34/35) | |||
| 3.0 | 653.94 | [M − SO3 + H]+ | (34/35) | |||
| 3.0 | 733.69 | [M + H]+ | (34/35) | |||
| 3.5 | 653.96 | [M − SO3 + H]+ | (34/35) | |||
| 3.5 | 733.60 | [M + H]+ | (34/35) | |||
| 6 | NIES-103 | 40 | 4.9 | 653.36 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) |
| 4.9 | 733.15 | [M + H]+ | (34/35) | |||
| 60 | 5.3 | 653.37 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) | ||
| 5.3 | 733.07 | [M + H]+ | (34/35) | |||
| 16.0 | 520.14 | [M + 2H]2+ | microcystin-RR (19) | |||
| 16.0 | 1038.61 | [M + H]+ | (19) | |||
| 18.6 | 523.39 | [M + 2H]2+ | microcystin-YR(20) | |||
| 18.6 | 1045.62 | [M + H]+ | (20) | |||
| 19.0 | 995.65 | [M + H]+ | microcystin-LR (18) | |||
| 7 | NIES-107 | 60 | 7.9 | 606.28 | [M + H]+ | aeruginosin 298A (32) |
| 15.9 | 520.10 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.9 | 1038.58 | [M + H]+ | (19) | |||
| 19.0 | 995.64 | [M + H]+ | microcystin-LR (18) | |||
| 8 | NIES-1025 | 40 | 2.3 | 653.33 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) |
| 4.9 | 733.14 | [M + H]+ | (34/35) | |||
| 60 | 5.0 | 653.34 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) | ||
| 5.0 | 733.13 | [M + H]+ | (34/35) | |||
| 15.8 | 520.15 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.8 | 1038.71 | [M + H]+ | microcystin-RR (19) | |||
| 19.0 | 995.71 | [M + H]+ | microcystin-LR (18) | |||
| 80 | 5.3 | 653.32 | [M − SO3 + H]+ | aeruginosin 102 (34/35) | ||
| 5.3 | 733.18 | [M + H]+ | (34/35) | |||
| 15.7 | 520.14 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.7 | 1038.71 | [M + H]+ | (19) | |||
| 19.0 | 995.69 | [M + H]+ | microcystin-LR (18) | |||
| 22.5 | 1029.71 | [M + H]+ | microcystin-FR (29) | |||
| 9 | NIES-1058 | 40 | 9.0 | 717.07 | [M + H]+ | aeruginosin 89A/B (26/27) |
| 10.7 | 717.07 | [M + H]+ | (26/27) | |||
| 60 | 8.9 | 717.13 | [M + H]+ | aeruginosin 89A/B (26/27) | ||
| 10.9 | 717.13 | [M + H]+ | (26/27) | |||
| 15.9 | 520.15 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.9 | 1038.60 | [M + H]+ | (19) | |||
| 18.5 | 1045.60 | [M + H]+ | microcystin-YR (20) | |||
| 10 | NIES-1071 | 60 | 8.5 | 637.40 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) |
| 8.5 | 717.12 | [M + H]+ | (26/27) | |||
| 9.0 | 637.35 | [M − SO3 + H]+ | (26/27) | |||
| 9.0 | 717.11 | [M + H]+ | (26/27) | |||
| 10.1 | 637.33 | [M − SO3 + H]+ | (26/27) | |||
| 10.1 | 717.09 | [M + H]+ | (26/27) | |||
| 10.4 | 637.33 | [M − SO3 + H]+ | (26/27) | |||
| 10.4 | 717.07 | [M + H]+ | (26/27) | |||
| 15.8 | 513.09 | [M + 2H]2+ | 7-desmethylmicrocystin-RR(28) | |||
| 15.8 | 1024.64 | [M + H]+ | (28) | |||
| 15.9 | 520.12 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.9 | 1038.60 | [M + H]+ | microcystin-RR (19) | |||
| 19.0 | 995.61 | [M + H]+ | microcystin-LR (18) | |||
| 11 | NIES-1085 | 60 | 9.5 | 637.33 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) |
| 11.1 | 717.18 | [M + H]+ | (26/27) | |||
| 12 | NIES-1099 | 60 | 8.4 | 637.44 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) |
| 8.4 | 717.09 | [M + H]+ | (26/27) | |||
| 10.0 | 637.38 | [M − SO3 + H]+ | (26/27) | |||
| 10.0 | 717.11 | [M + H]+ | (26/27) | |||
| 10.3 | 637.36 | [M − SO3 + H]+ | (26/27) | |||
| 10.3 | 717.14 | [M + H]+ | (26/27) | |||
| 15.8 | 520.11 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.8 | 1038.60 | [M + H]+ | (19) | |||
| 18.4 | 1045.58 | [M + H]+ | microcystin-YR(20) | |||
| 13 | NIES-1133 | 40 | 1.8 | 653.41 | [M − SO3 + H]+ | aeruginosin 102A/B (34/35) |
| 1.8 | 733.14 | [M + H]+ | (34/35) | |||
| 2.3 | 653.44 | [M − SO3 + H]+ | (34/35) | |||
| 2.3 | 733.13 | [M + H]+ | (34/35) | |||
| 3.2 | 653.37 | [M − SO3 + H]+ | (34/35) | |||
| 3.2 | 733.14 | [M + H]+ | (34/35) | |||
| 3.7 | 653.39 | [M − SO3 + H]+ | (34/35) | |||
| 3.7 | 733.11 | [M + H]+ | (34/35) | |||
| 4.0 | 653.35 | [M − SO3 + H]+ | (34/35) | |||
| 4.0 | 733.09 | [M + H]+ | (34/35) | |||
| 60 | 9.0 | 637.39 | [M − SO3 + H]+ | aeruginosin 89A/B (26/27) | ||
| 9.9 | 637.39 | [M − SO3 + H]+ | (26/27) | |||
| 9.9 | 717.34 | [M + H]+ | (26/27) | |||
| 15.6 | 520.16 | [M + 2H]2+ | microcystin-RR (19) | |||
| 15.6 | 1038.56 | [M + H]+ | (19) | |||
| 18.9 | 498.44 | [M + 2H]2+ | microcystin-LR (18) | |||
| 18.9 | 995.70 | [M + H]+ | (18) | |||
| 14 | NIES-1043 | 40 | 6.4 | 733.12 | [M + H]+ | aeruginosin 102A/B (34/35) |
| 10.4 | 717.03 | [M + H]+ | aeruginosin 89A/B (26/27) | |||
| 10.4 | 733.86 | [M + H]+ | (34/35) | |||
| 15 | NIES-298 | 40 | 7.1 | 605.47 | [M + H]+ | aeruginosin 298A (32) |
| 7.1 | 621.40 | [M + H2O + H]+ | aeruginosin K139 (30) | |||
| 60 | 6.6 | 603.37 | [M + H]+ | aeruginosin K139 (30) | ||
| 7.4 | 621.40 | [M + H2O + H]+ | (30) | |||
| 80 | 6.6 | 603.35 | [M + H]+ | aeruginosin K139 (30) | ||
| 6.6 | 621.38 | [M + H2O + H]+ | (30) |



3. Experimental Section
3.1. Culture Condition
3.2. Extraction
3.3. Serine Protease Inhibitory Assays
3.3.1. Thrombin Inhibitory Assay
3.3.2. FVIIa and FVIIa-sTF Assays
Preparation of l-α-Cephalin or 3-sn-Phosphatidylethanolamine Buffer
FVIIa Assay
FVIIa-sTF Assay
Preparation of FVIIa Enzyme
Preparation of Soluble Tissue Factor (sTF or F3-28H)
3.4. FVIIa-sTF Assay Procedure
3.5. LC-MS Preparation of Samples and Determination of fVIIa-sTF Active Compounds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davie, E.W. A brief historical review of the waterfall/cascade of blood coagulation. J. Biol. Chem. 2003, 278, 50819–50832. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K.; Kurachi, K.; Kisiel, W. The role of serine proteases in the blood coagulation cascade. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 48, 277–318. [Google Scholar] [PubMed]
- Davie, E.W.; Ratnoff, O.D. Waterfall sequence for intrinsic blood clotting. Science 1964, 145, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Scandura, J.M.; Ahmad, S.S.; Walsh, P.N. A binding site expressed on the surface of activated human platelets is shared by factor X and prothrombin. Biochemistry 1996, 35, 8890–8902. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.L.; Desai, U.R. Recent research developments in the direct inhibition of coagulation proteinases-inhibitors of the initiation phase. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.S.; Roberge, M.; Quan, C.; Lazarus, R.A. Selection and characterization of a new class of peptide exosite inhibitors of coagulation factor VIIa. Biochemistry 2001, 40, 9513–9521. [Google Scholar] [CrossRef] [PubMed]
- Weston, B.W.; Monahan, P.E. Familial deficiency of vitamin K-dependent clotting factors. Haemophilia 2008, 14, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.; Hirsh, J.; Hylek, E.; Jacobson, A.; Crowther, M.; Palareti, G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2008, 133 (Suppl. 6), 160S–198S. [Google Scholar] [CrossRef] [PubMed]
- Chlipala, G.E.; Mo, S.; Orjala, J. Chemodiversity in freshwater and terrestrial cyanobacteria—A source for drug discovery. Curr. Drug Targets 2011, 12, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Fujii, K.; Shimada, T.; Suzuki, M. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos.aquae NRC 525-17. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Murakami, M.; Ishida, K.; Okino, T.; Okita, Y.; Matsuda, H.; Yamaguchi, K. Aeruginosins 98-A and B, trypsin inhibitors from the blue-green alga Microcystis aeruginosa NIES-98. Tetrahedron Lett. 1995, 36, 2785–2788. [Google Scholar] [CrossRef]
- Ishida, K.; Okita, Y.; Matsuda, H.; Okino, T.; Murakami, M. Aeruginosins, protease inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron 1999, 55, 10971–10988. [Google Scholar] [CrossRef]
- Adiv, S.; Carmeli, S. Protease inhibitors from Microcystis aeruginosa bloom material collected from the Dalton Reservoir, Israel. J. Nat. Prod. 2013, 76, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Murakami, M.; Haraguchi, R.; Munekata, H.; Matsuda, H.; Yamaguchi, K. Micropeptins A and B, plasmin and trypsin inhibitors from the blue-green alga Microcystis aeruginosa. Tetrahedron Lett. 1993, 34, 8131–8134. [Google Scholar] [CrossRef]
- Gesner-Apter, S.; Carmeli, S. Protease inhibitors from a water bloom of the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 2009, 72, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Adiv, S.; Aharonv-Nadborny, R.; Carmeli, S. Micropeptins from Microcystis aeruginosa collected in Dalton reservoir, Israel. Tetrahedron 2010, 66, 7429–7436. [Google Scholar] [CrossRef]
- Harada, K.I.; Mayumi, T.; Shimada, T.; Fujii, K.; Kondo, F.; Park, H.D.; Watanabe, M.F. Co-production of microcystins and aeruginopeptins by natural cyanobacterial bloom. Environ. Toxicol. 2001, 16, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.R.J.; Harada, K.-I. Evaluation of serine protease inhibitors as potent fVIIa-sTF inhibitors in the blood coagulation cascade. Lett. Drug Des. Discov. 2016, 13, 3–23. [Google Scholar] [CrossRef]
- Palareti, G.; Leali, N.; Coccheri, S.; Poggi, M.; Manotti, C.; D'Angelo, A.; Pengo, V.; Erba, N.; Moia, M.; Ciavarella, N.; Devoto, G.; Berrettini, M.; Musolesi, S. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study (ISCOAT). Lancet 1996, 348, 423–428. [Google Scholar] [CrossRef]
- López, J.A.; Kearon, C.; Lee, A.Y. Deep venous thrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2004, 1, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.R.J.; Kisugi, T.; Umezawa, T.; Matsuda, F.; Campitelli, M.R.; Quinn, R.J.; Okino, T. Thrombin inhibitors from the freshwater cyanobacterium Anabaena compacta. J. Nat. Prod. 2012, 75, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Østerud, B.; Bjørklid, E. Sources of tissue factor. In Seminars in Thrombosis and Hemostasis; Stratton Intercontinental Medical Book Corporation c1974: New York, NY, USA, 2006; pp. 11–23. [Google Scholar]
- Nishizawa, A.; Arshad, A.B.; Nishizawa, T.; Asayama, M.; Fujii, K.; Nakano, T.; Harada, K.-I.; Shirai, M. Cloning and characterization of a new hetero-gene cluster of nonribosomal peptide synthetase and polyketide synthase from the cyanobacterium Microcystis aeruginosa K-139. J. Gen. Appl. Microbiol. 2007, 53, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.I.; Nakano, T.; Fujii, K.; Shirai, M. Comprehensive analysis system using liquid chromatography–mass spectrometry for the biosynthetic study of peptides produced by cyanobacteria. J. Chromatogr. A 2004, 1033, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Harada, K.I. Meijo University: Nagoya, Japan, Unpublished work. 2003.
- Welker, M.; Maršálek, B.; Šejnohová, L.; Von Doehren, H. Detection and identification of oligopeptides in Microcystis (cyanobacteria) colonies: Toward an understanding of metabolic diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- BioDataFit, version 1.02; software for EC50 calculation; Chang BioScience Inc.: Castro Valley, CA, USA, 2002–2013.
- Kadono, S. Selective inhibition mechanism of factor VIIa/tissue factor revealed from X-ray crystal structure analysis. J. Crystallogr. Soc. Jpn. 2006, 48, 147–152. [Google Scholar] [CrossRef]
- Hagen, F.S.; Gray, C.L.; O'Hara, P.; Grant, F.J.; Saari, G.C.; Woodbury, R.G.; Hart, C.E.; Insley, M.; Kisiel, W.; Kurachi, K.; Davie, E.W. Characterization of a cDNA coding for human factor VII. Proc. Natl. Acad. Sci. USA 1986, 83, 2412–2416. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Spencer, F.A. Thrombin: Structure, biochemistry, measurement, and status in clinical medicine. J. Thromb. Thrombolysis 1998, 5, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Konetschny-Rapp, S.; Krell, H.-W.; Martin, U. Isolation of Oscillarin. PCT WO96/11941, 1996. [Google Scholar]
- Ersmark, K.; del Valle, J.R.; Hanessian, S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. Engl. 2008, 47, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Buchanan, M.S.; Edser, A.; Hyde, E.; Simpson, M.; Quinn, R.J. Dysinosins B–D, inhibitors of factor VIIa and thrombin from the Australian sponge Lamellodysidea chlorea. J. Nat. Prod. 2004, 67, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Pierens, G.K.; Fechner, G.; de Almeida Leone, P.; Ngo, A.; Simpson, M.; Hyde, E.; Hooper, J.N.A.; Boström, S.-L.; Musil, D.; Quinn, R.J. Dysinosin A: A novel inhibitor of Factor VIIa and thrombin from a new genus and species of Australian sponge of the family Dysideidae. J. Am. Chem. Soc. 2002, 124, 13340–13341. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Del Valle, J.R.; Xue, Y.; Blomberg, N. Total synthesis and structural confirmation of chlorodysinosin A. J. Am. Chem. Soc. 2006, 128, 10491–10495. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Sivonen, K.; Adachi, K.; Noguchi, K.; Sano, H.; Hirayama, K.; Suzuki, M.; Harada, K.-I. Comparative study of toxic and non-toxic cyanobacterial products: Novel peptides from toxic Nodularia spumigena AV1. Tetrahedron Lett. 1997, 38, 5525–5528. [Google Scholar] [CrossRef]
- Goyal, N. Synthesis and Biological Evaluation of Aeruginosin Based Compounds and Self-Assembly of Glucosamine Based Compounds. Ph.D. Thesis, University of New Orleans, New Orleans, LA, USA, 2011. [Google Scholar]
- Kasai, F.; Kawachi, M.; Erata, M.; Watanabe, M.M. NIES Collection: List of Strains, 7th ed.National Institute for Environmental Studies: Tsukuba, Japan, 2004; Volume 182.
- Kaplan, N.P.; Colowick, N.P.; Laszlo, L. Proteolytic enzymes Part B. In Methods in Enzymology; Academic Press: New York, NY, USA, 1976; Volume XLV. [Google Scholar]
- Shin, H.J.; Murakami, M.; Matsuda, H.; Yamaguchi, K. Microviridins D–F, serine protease inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-204). Tetrahedron 1996, 52, 8159–8168. [Google Scholar] [CrossRef]
- Nakagura, T.; Tabata, K.; Kira, K.; Hirota, S.; Clark, R.; Matsuura, F.; Hiyoshi, H. Selective tissue factor/factor VIIa Inhibitor, ER-410660, and its prodrug, E5539, have anti-venous and anti-arterial thrombotic effects with a low risk of bleeding. Thromb. Res. 2013, 13, 271–279. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anas, A.R.J.; Nakajima, A.; Naruse, C.; Tone, M.; Asukabe, H.; Harada, K.-i. Determination of FVIIa-sTF Inhibitors in Toxic Microcystis Cyanobacteria by LC-MS Technique. Mar. Drugs 2016, 14, 7. https://doi.org/10.3390/md14010007
Anas ARJ, Nakajima A, Naruse C, Tone M, Asukabe H, Harada K-i. Determination of FVIIa-sTF Inhibitors in Toxic Microcystis Cyanobacteria by LC-MS Technique. Marine Drugs. 2016; 14(1):7. https://doi.org/10.3390/md14010007
Chicago/Turabian StyleAnas, Andrea Roxanne J., Anna Nakajima, Chiaki Naruse, Mineka Tone, Hirohiko Asukabe, and Ken-ichi Harada. 2016. "Determination of FVIIa-sTF Inhibitors in Toxic Microcystis Cyanobacteria by LC-MS Technique" Marine Drugs 14, no. 1: 7. https://doi.org/10.3390/md14010007
APA StyleAnas, A. R. J., Nakajima, A., Naruse, C., Tone, M., Asukabe, H., & Harada, K. -i. (2016). Determination of FVIIa-sTF Inhibitors in Toxic Microcystis Cyanobacteria by LC-MS Technique. Marine Drugs, 14(1), 7. https://doi.org/10.3390/md14010007