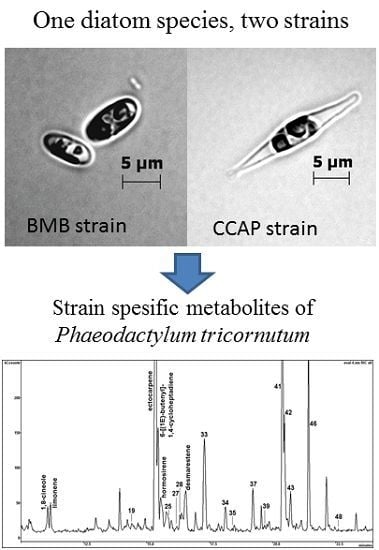
Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water
Abstract
:1. Introduction
2. Results
2.1. Different Growth and Protein Expression of BMB and CCAP Strains



| Strain | Day | Total Soluble Protein, pg/Cell | Resolved Protein Spots by 2DGE |
|---|---|---|---|
| BMB | 2 | 2.56 ± 0.45 | 197 ± 11 |
| 4 | 0.57 ± 0.06 | 289 ± 18 | |
| 8 | 0.25 ± 0.04 | 198 ± 19 | |
| CCAP | 2 | 3.12 ± 0.30 | 372 ± 41 |
| 4 | 1.10 ± 0.10 | 242 ± 39 | |
| 8 | 0.13 ± 0.02 | 90 ± 6 |
2.2. Fatty Acid Content and Composition of BMB and CCAP Strains

2.3. Differences in Volatile Organic Compounds (VOCs) from BMB and CCAP Strains
| No. | RI # | COMPOUND | BMB-E-0007; % of Total | CCAP 1052/1A; % of Total | MS Fragments |
|---|---|---|---|---|---|
| 1 | 516 | dimethyl sulfide | 2.91 | 7.82 | |
| 2 | 746 | dimethyl disulfide | 0.42 | - | |
| 3 | 792 | 2-hexanone | - | 2.31 | |
| 4 | 867 | p-xylene | - | 1,40 | |
| 5 | 941 | α-pinene | 0.06 | 0.84 | |
| 6 | 966 | dimethyl trisulfide | 0.25 | - | |
| 7 | 983 | 6-methyl-5-hepten-2-one | 0.10 | 2.52 | |
| 8 | 1017 | 2,2,6-trimethyl-cyclohexanone | 0.13 | 1.12 | |
| 9 | 1027 | 1,8-cineole | 0.27 | 5.42 | |
| 10 | 1029 | limonene | 0.88 | 6.47 | |
| 11 | 1037 | acetophenone | - | 3.81 | |
| 12 | 1060 | 3-methyl decane | - | 0.47 | |
| 13 | 1070 | aliphatic hydrocarbon | 0.11 | 5.68 | m/z = 43, 57, 71, 85, 97, 111, 125 |
| 14 | 1077 | aliphatic hydrocarbon | - | 1.62 | m/z = 43, 57, 71, 85, 98, 111, 123 |
| 15 | 1080 | 3-acetyl-2,5-dimethyl furan | 0.17 | - | |
| 16 | 1099 | nonanal | 0.19 | 1.52 | |
| 17 | 1100 | undecane | 0.39 | 1.31 | |
| 18 | 1126 | 4-oxoisophorone | 0.17 | 6.27 | |
| 19 | 1133 | putative pheromonal VOC | 1.12 | - | m/z = 146(10), 117(100), 91(70), 104(45), 115(50) |
| 20 | 1134 | aliphatic hydrocarbon | - | 1.99 | m/z = 43, 57, 71, 85, 113 |
| 21 | 1141 | aromatic compound | 0.07 | 5.78 | |
| 22 | 1163 | ectocarpene | 5.41 | - | |
| 23 | 1166 | 6-((1E)-butenyl)-1,4-cycloheptadiene | 58.52 | - | |
| 24 | 1169 | hormosirene | 0.21 | - | |
| 25 | 1176 | putative pheromonal VOC | 2.24 | - | m/z = 148(10), 91(100), 79(50), 119(40), 105(35) |
| 26 | 1179 | aromatic compound | 0.25 | 1.72 | m/z = 122(100), 107(95), 77(78), 91(36) |
| 27 | 1185 | putative pheromonal VOC | 0.63 | - | m/z = 148(5), 91(100), 79(90), 105(45) |
| 28 | 1187 | putative pheromonal VOC | 0.92 | - | m/z = 146(45), 117(100), 91(90), 131(90), 115(75) |
| 29 | 1190 | octanoic acid | 0.07 | 0.95 | |
| 30 | 1192 | desmarestene | 1.01 | - | |
| 31 | 1203 | decanal | 0.18 | 1.52 | |
| 32 | 1209 | β-cyclocitral | 0.06 | 1.57 | |
| 33 | 1213 | putative pheromonal VOC | 2.30 | - | m/z = 148(50), 91(100), 105(60), 119(45) |
| 34 | 1237 | putative pheromonal VOC | 0.34 | - | m/z = 146(40), 91(100), 117(90), 131(40) |
| 35 | 1246 | putative pheromonal VOC | 0.12 | - | m/z = 148(45), 91(100), 79(50), 105(40), 119(40) |
| 36 | 1249 | aromatic compound | 0.14 | - | m/z = 148(25), 122(100), 107(85), 91(50), 77(45) |
| 37 | 1273 | putative pheromonal VOC | 0.40 | - | m/z = 148(50), 91(100), 105(50), 119(45), 77(40) |
| 38 | 1284 | nonanoic acid | 0.15 | 3.78 | |
| 39 | 1291 | putative pheromonal VOC | 0.04 | - | m/z = 162(17), 147(100), 119(60), 91(25) |
| 40 | 1300 | tridecane | 0.10 | 4.99 | |
| 41 | 1310 | putative pheromonal VOC | 9.43 | - | m/z = 162(3), 147(2), 91(100), 79(25), 105(22) |
| 42 | 1313 | putative pheromonal VOC | 0.60 | - | m/z = 148(7), 79(100), 91(70), 67(65), 135(25) |
| 43 | 1322 | putative pheromonal VOC | 0.62 | - | m/z = 146(15), 79(10), 91(80), 67(60), 53(40) |
| 44 | 1327 | aliphatic hydrocarbon | 0.98 | - | m/z = 43, 57, 71, 85 |
| 45 | 1335 | cyclo-β-ionone | 0.23 | 1.69 | |
| 46 | 1346 | putative pheromonal VOC | 2.24 | - | m/z = 166(20), 67(100), 81(60), 95(55), 109(50) |
| 47 | 1382 | decanoic acid | 0.04 | 1.37 | |
| 48 | 1388 | putative pheromonal VOC | 0.31 | - | m/z = 164(30), 91(95), 79(70), 105(55) |
| 49 | 1410 | dodecanal | 0.16 | 1.84 | |
| 50 | 1449 | geranyl acetone | 0.24 | 4.47 | |
| 51 | 1460 | 2,6-di-tert-butyl-p-benzoquinone | 0.18 | 1.84 | |
| 52 | 1504 | 2,4-di-tert-butyl phenol | 0.07 | 0.98 | |
| 53 | 1558 | butyl decanoate | 0.88 | 3.05 | |
| 54 | 1592 | tetradecanal | 0.10 | 1.38 | |
| 55 | 1595 | dodecanoic acid | 0.03 | 1.11 | |
| 56 | 1815 | (E,E)-farnesyl acetate | 0.16 | 1.43 | |
| 57 | 2000 | eicosane | 0.30 | - | |
| 58 | 2178 | ethyl hexadecanoate | 0.44 | 2.67 | |
| 59 | 2200 | docosane | 0.05 | 6.14 | |
| 60 | 2832 | squalene | 2.24 | - | |
| 61 | 3000 | triacontane | 0.34 | 1.13 | |
| sum % | 100 | 100 | |||
| Total MS detector response | 1.56E + 07 | 1.28E + 06 |

3. Discussion
4. Experimental Section
4.1. Cultivation of BMB and CCAP Strains for Growth and Proteins Analyses
4.2. Growth Measurements
4.3. Preparation of Protein Samples
4.4. Two Dimensional Gel Electrophoresis (2DGE)
4.5. Visualization, Imaging and Analysis of Gels
4.6. Cultivation of Strains for Determination of Total Fatty Acids and Profiling
4.7. Fatty Acid Extraction
4.8. FAME Analysis by Gas Chromatography and Mass Spectrometry (GC-MS)
4.9. Sampling of Volatiles by Headspace Solid-Phase Microextraction (SPME)
4.10. Volatile Analysis by Gas Chromatography and Mass Spectrometry (GC-MS)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Medlin, L.K.; Kooistra, W.C.H.F.; Schmid, A.M.M. A review of the evolution of diatoms: A total approach using molecules, morphology and geology. In The Origin and Early Evolution of the Diatoms; Witkowski, A., Sieminska, J., Eds.; Polish Academy of Sciences: Cracow, Poland, 2000; pp. 13–35. [Google Scholar]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.G. Patterns of sexual reproduction in diatoms. Hydrobiologia 1993, 269, 11–20. [Google Scholar] [CrossRef]
- Stonik, V.; Stonik, I. Low molecular weight metabolites from diatoms: Structures, biological roles and biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Kroth, P.G.; Chiovatti, A.; Gruber, A.; Martin-Jezequel, V.; Moch, T.; Parker, M.S.; Stanley, M.S.; Kaplan, A.; Caron, L.; Weber, T.; et al. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 2008, 3, e1426. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; LaRoche, J.; Maheswari, U.; Lommer, M.; Schauer, N.; Lopez, P.J.; Finazzi, G.; Fernie, A.R.; Bowler, C. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc. Natl. Acad. Sci. USA 2008, 105, 10438–10443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohnert, G.; Boland, W. Biosynthesis of the algal pheromone hormosirene by the freshwater diatom Gomphonema parvulum (Bacillariophyceae). Tetrahedron 1996, 52, 10073–10082. [Google Scholar] [CrossRef]
- Pohnert, G.; Lumineau, O.; Cueff, A.; Adolph, S.; Cordevant, C.; Lange, M.; Poulet, S. Are volatile unsaturated aldehydes from diatoms the main line of chemical defence against copepods? Mar. Ecol. Prog. Ser. 2002, 245, 33–45. [Google Scholar] [CrossRef]
- Barofsky, A.; Vidoudez, C.; Pohnert, G. Metabolic profiling reveals growth stage variability in diatom exudates. Limnol. Oceanogr. Meth. 2009, 7, 382–390. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [PubMed]
- Schobert, B.; Elstner, E.F. Production of hexanal and ethane by Phaeodactylum triconutum and its correlation to fatty-acid oxidation and bleaching of photosynthetic pigments. Plant Physiol. 1980, 66, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Yongmanitchai, W.; Ward, O.P. Growth of and omega-3-fatty-acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [PubMed]
- Atalah, E.; Cruz, C.M.H.; Izquierdo, M.S.; Rosenlund, G.; Caballero, M.J.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar] [CrossRef]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.H.; Bowler, C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Johansen, J.R. Morphological variability and cell-wall composition of Phaeodactylum tricornutum (Bacillariophyceae). Great Bas. Nat. 1991, 51, 310–315. [Google Scholar]
- Lewin, J.C.; Lewin, R.A.; Philpott, D.E. Observations on Phaeodactylum tricornutum. J. Gen. Microbiol. 1958, 18, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Volcani, B.E. Polymorphic diatom Phaeodactylum tricornutum ultrastructure of its morphotypes. J. Phycol. 1978, 14, 10–21. [Google Scholar] [CrossRef]
- He, L.Y.; Han, X.T.; Yu, Z.M. A rare Phaeodactylum tricornutum cruciform morphotype: Culture conditions, transformation and unique fatty acid characteristics. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Lewin, J.C. The taxonomic position of Phaeodactylum tricornutum. J. Gen. Microbiol. 1958, 18, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: New York, USA, 1990; p. 560. [Google Scholar]
- Prestegard, S.; Oftedal, L.; Coyne, R.T.; Nygaard, G.; Skjærven, K.H.; Knutsen, G.; Døskeland, S.O.; Herfindal, L. Marine benthic diatoms contain compounds able to induce leukemia cell death and modulate blood platelet activity. Mar. Drugs 2009, 7, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Prestegard, S.K.; Knutsen, G.; Herfindal, L. Adenosine content and growth in the diatom Phaeodactylum tricornutum (Bacillariophyceae): Effect of salinity, light, temperature and nitrate. Diatom Res. 2014, 29, 361–369. [Google Scholar] [CrossRef]
- Hildebrand, M.; Dahlin, K. Nitrate transporter genes from the diatom Cylindrotheca fusiformis (Bacillariophyceae): mRNA levels controlled by nitrogen source and by the cell cycle. J. Phycol. 2000, 36, 702–713. [Google Scholar] [CrossRef]
- Veluchamy, A.; Lin, X.; Maumus, F.; Rivarola, M.; Bhavsar, J.; Creasy, T.; O’Brien, K.; Sengamalay, N.A.; Tallon, L.J.; Smith, A.D.; et al. Insights into the role of DNA methylation in diatoms by genome-wide profiling in Phaeodactylum tricornutum. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T.; Ward, A.C.; Goodfellow, M. Search and discovery strategies for biotechnology: The paradigm shift. Microbiol. Mol. Biol. Rev. 2000, 64, 573–606. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-cell repsonse to nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 2015. [Google Scholar] [CrossRef] [PubMed]
- Quigg, A.; Beardall, J. Protein turnover in relation to maintenance metabolism at low photon flux in two marine microalgae. Plant Cell Environ. 2003, 26, 693–703. [Google Scholar] [CrossRef]
- Chan, L.L.; Hodgkiss, I.J.; Wan, J.M.F.; Lum, J.H.K.; Mak, A.S.C.; Sit, W.H.; Lo, S.C.L. Proteomic study of a model causative agent of harmful algal blooms, Prorocentrum triestinum II: The use of differentially expressed protein profiles under different growth phases and growth conditions for bloom prediction. Proteomics 2004, 4, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.J.; Laroche, J.; Greene, R.M.; Olaizola, M. Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation. J. Phycol. 1993, 29, 755–766. [Google Scholar] [CrossRef]
- Gutenbrunner, S.A.; Thalhamer, J.; Schmid, A.M.M. Proteinaceous and immunochemical distinctions between the oval and fusiform morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 1994, 30, 129–136. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Berge, J.P.; del Pino, V.; Uronen, P.; Legrand, C. Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef]
- Desbois, A.P.; Walton, M.; Smith, V.J. Differential antibacterial activities of fusiform and oval morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). J. Mar. Biol. Assoc. UK 2010, 90, 769–774. [Google Scholar] [CrossRef]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour. Technol. 2012, 124, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Beardall, J.; Heraud, P. Changes in growth, chlorophyll fluorescence and fatty acid composition with culture age in batch cultures of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Bot. Mar. 2006, 49, 165–173. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lebl, T.; Yan, L.M.; Smith, V.J. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wendel, T.; Jüttner, F. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms. Phytochemistry 1996, 41, 1445–1449. [Google Scholar] [CrossRef]
- Derenbach, J.B.; Pesando, D. Investigations into a small fraction of volatile hydrocarbons III. Two diatom cultures produce ectocarpene, a pheromone of brown algae. Mar. Chem. 1986, 19, 337–341. [Google Scholar] [CrossRef]
- Stratmann, K.; Boland, W.; Müller, D.G. Biosynthesis of pheromones in female gametes of marine brown algae (Phaeophyceae). Tetrahedron 1993, 49, 3755–3766. [Google Scholar] [CrossRef]
- Kodama, K.; Matsui, K.; Hatanaka, A.; Kajiwara, T. Sex-attractants secreted from female gametes of Japanese brown algae of the genus Scytosiphon. Phytochemistry 1993, 32, 817–819. [Google Scholar] [CrossRef]
- Müller, D.G.; Kawai, H.; Stache, B.; Fölster, E.; Boland, W. Sexual pheromones and gamete chemotaxis in Analipus japonicus (Phaeophyceae). Experientia 1990, 46, 534–536. [Google Scholar] [CrossRef]
- Maier, I.; Müller, D.G. Sexual pheromones in algae. Biol. Bull. 1986, 170, 145–175. [Google Scholar] [CrossRef]
- Schnitzler, I.; Pohnert, G.; Hay, M.; Boland, W. Chemical defense of brown algae (Dictyopteris spp.) against the herbivorous amphipod Ampithoe longimana. Oecologia 2001, 126, 515–521. [Google Scholar] [CrossRef]
- Marshall Darley, W. Deoxyribonucleic acid content of the three cell types of Phaeodactylum tricornutum Bohlin. J. Phycol. 1968, 4, 219–220. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Yassaa, N.; Peeken, I.; Zollner, E.; Bluhm, K.; Arnold, S.; Spracklen, D.; Williams, J. Evidence for marine production of monoterpenes. Environ. Chem. 2008, 5, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Kamenarska, Z.; Ivanova, A.; Stancheva, R.; Stoyneva, M.; Stefanov, K.; Dimitrova-Konaklieva, S.; Popov, S. Volatile compounds from some black sea red algae and their chemotaxonomic application. Bot. Mar. 2006, 49, 47–56. [Google Scholar] [CrossRef]
- Vogt, M.; Turner, S.; Yassaa, N.; Steinke, M.; Williams, J.; Liss, P. Laboratory inter-comparison of dissolved dimethyl sulphide (DMS) measurements using purge-and-trap and solid-phase microextraction techniques during a mesocosm experiment. Mar. Chem. 2008, 108, 32–39. [Google Scholar] [CrossRef]
- Liss, P.S.; Hatton, A.D.; Malin, G.; Nightingale, P.D.; Turner, S.M. Marine sulphur emissions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 352, 159–168. [Google Scholar] [CrossRef]
- Abrahamsson, K.; Choo, K.S.; Pedersen, M.; Johansson, G.; Snoeijs, P. Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae. Phytochemistry 2003, 64, 725–734. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.E.; Parks, L.W.; Spence, K.D. Some effects of Douglas fir terpenes on certain microorganisms. Appl. Envir. Microbiol. 1980, 40, 301–304. [Google Scholar]
- Uribe, S.; Ramirez, J.; Pena, A. Effects of beta-pinene on yeast membrane functions. J. Bacteriol. 1985, 161, 1195–1200. [Google Scholar] [PubMed]
- Hismiogullari, S.E.; Elyurek, E.; Hismiogullari, A.A.; Sahin, F.; Basalan, M.; Yenice, S. Effects of caproic and caprylic acids on microbial growth and cytotoxicity. J. Anim. Vet. Adv. 2008, 7, 731–735. [Google Scholar]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Pohnert, G. Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol. 2002, 129, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.T.; Li, Y.; Nowak, E.; Schenk, P.M. Microalgae isolation and selection for prospective biodiesel production. Energies 2012, 5, 1835–1849. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Romano, G.; Russo, G.L.; Buttino, I.; Ianora, A.; Miralto, A. A marine diatom-derived aldehyde induces apoptosis in copepod and sea urchin embryos. J. Exp. Biol. 2003, 206, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Teratogens as anti-cancer drugs. Cell Cycle 2005, 4, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Walne, P.R. Studies on the Food Value of Nineteen Genera of Algae to Juvenile Bivalves of the Genera Ostrea, Crassostrea, Mercenaria and Mytilus; H.M. Stationery Office: London, UK, 1970; Volume 26, pp. 1–62. [Google Scholar]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of artificial seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Wang, S.B.; Hu, Q.; Sommerfeld, M.; Chen, F. An optimized protocol for isolation of soluble proteins from microalgae for two-dimensional gel electrophoresis analysis. J. Appl. Phycol. 2003, 15, 485–496. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Meier, S.; Mjos, S.A.; Joensen, H.; Grahl-Nielsen, O. Validation of a one-step extraction/methylation method for determination of fatty acids and cholesterol in marine tissues. J. Chromatogr. A 2006, 1104, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wasta, Z.; Mjos, S.A. A database of chromatographic properties and mass spectra of fatty acid methyl esters from omega-3 products. J. Chromatogr. A 2013, 1299, 94–102. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestegard, S.K.; Erga, S.R.; Steinrücken, P.; Mjøs, S.A.; Knutsen, G.; Rohloff, J. Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water. Mar. Drugs 2016, 14, 9. https://doi.org/10.3390/md14010009
Prestegard SK, Erga SR, Steinrücken P, Mjøs SA, Knutsen G, Rohloff J. Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water. Marine Drugs. 2016; 14(1):9. https://doi.org/10.3390/md14010009
Chicago/Turabian StylePrestegard, Siv Kristin, Svein Rune Erga, Pia Steinrücken, Svein Are Mjøs, Gjert Knutsen, and Jens Rohloff. 2016. "Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water" Marine Drugs 14, no. 1: 9. https://doi.org/10.3390/md14010009
APA StylePrestegard, S. K., Erga, S. R., Steinrücken, P., Mjøs, S. A., Knutsen, G., & Rohloff, J. (2016). Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water. Marine Drugs, 14(1), 9. https://doi.org/10.3390/md14010009