Exploitable Lipids and Fatty Acids in the Invasive Oyster Crassostrea gigas on the French Atlantic Coast
Abstract
:1. Introduction
2. Results and Discussion
2.1. Seasonal Lipid Content and Lipid Class Composition
2.2. Seasonal Phospholipid Class Composition
2.3. Seasonal Phospholipid Fatty Acid Composition
2.4. Free Sterol Composition
3. Materials and Methods
3.1. Specimen Collection
3.2. Chemicals
3.3. Lipid Analyses
3.3.1. Total Lipid Extraction and Separation of Lipid Classes
3.3.2. High Performance Liquid Chromatography (HPLC) Analyses of Phospholipids
3.3.3. Gas Chromatography-Mass Spectrometry (GC-MS)
3.4. Data Presentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAEP | ceramide aminoethylphosphonate |
| DHA | n-3 docosahexaenoic acid |
| DMA(s) | dimethylacetal(s) |
| DW | dry weight |
| EPA | n-3 eicosapentaenoic acid |
| ELSD | evaporative light scattering |
| FA(s) | fatty acid(s) |
| FAME(s) | fatty acid methyl ester(s) |
| GC-MS | gas chromatography-mass spectrometry |
| HPLC | high performance liquid chromatography |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| MUFA(s) | monounsaturated fatty acid(s) |
| NAP(s) | N-acyl pyrrolidide(s) |
| NMID | non-methylene-interrupted dienoic |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| PUFA(s) | polyunsaturated fatty acid(s) |
| PL(s) | phospholipid(s) |
| SA(s) | sterol acetate(s) |
| SFA(s) | saturated fatty acid(s) |
| SPH | sphingomyelin |
| TL | total lipids |
References
- Grizel, H.; Héral, M. Introduction into France of the Japanese oyster (Crassostrea gigas). J. Cons. ICES J. Mar. Sci. 1991, 47, 399–403. [Google Scholar] [CrossRef]
- Ruesink, J.L.; Lenihan, H.S.; Trimble, A.C.; Heiman, K.W.; Micheli, F.; Byers, J.E.; Kay, M.C. Introduction of non-native oysters: Ecosystem effects and restoration Implications. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 643–689. [Google Scholar] [CrossRef]
- Cognie, B.; Haure, J.; Barillé, L. Spatial distribution in a temperate coastal ecosystem of the wild stock of the farmed oyster Crassostrea gigas (Thunberg). Aquaculture 2006, 259, 249–259. [Google Scholar] [CrossRef]
- Troost, K. Causes and effects of a highly successful marine invasion: Case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. J. Sea Res. 2010, 64, 145–165. [Google Scholar] [CrossRef]
- Dutertre, M.; Beninger, P.G.; Barillé, L.; Papin, M.; Haure, J. Rising water temperatures, reproduction and recruitment of an invasive oyster, Crassostrea gigas, on the French Atlantic coast. Mar. Environ. Res. 2010, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Thouzeau, G. Marine species introduced on the French Channel-Atlantic coasts: A review of main biological invasions and impacts. Open J. Ecol. 2015, 5, 227–257. [Google Scholar] [CrossRef]
- Martin, J.-L.; Haure, J.; Dupuy, B.; Nourry, M.; Palvadeau, H.; Papin, M.; Penisson, C.; Thouard, E. Estimation de Stocks D’huîtres Sauvages sur les Zones Concédées de la Partie Vendéenne de la Baie de Bourgneuf en 2003; DRV/RA/LCPL/2004-03; Ifremer: Issy-Les-Moulineaux, France, 2004. [Google Scholar]
- Martin, J.-L.; Haure, J.; Dupuy, B.; Nourry, M.; Palvadeau, H.; Papin, M.; Penisson, C.; Thouard, E. Estimation des Stocks D’huîtres Sauvages sur les Zones non Concédées de la Partie Vendéenne de la Baie de Bourgneuf en 2004; AGS/LGP/Bouin/2005-01; Ifremer: Issy-Les-Moulineaux, France, 2005. [Google Scholar]
- Dagorn, F.; Buzin, F.; Couzinet-Mossion, A.; Decottignies, P.; Viau, M.; Rabesaotra, V.; Barnathan, G.; Wielgosz-Collin, G. Multiple beneficial lipids including lecithin detected in the edible invasive mollusk Crepidula fornicata from the French Northeastern Atlantic coast. Mar. Drugs 2014, 12, 6254–6268. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. Int. Rev. J. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Bergé, J.-P.; Barnathan, G. Fatty Acids from Lipids of Marine Organisms: Molecular Biodiversity, Roles as Biomarkers, Biologically Active Compounds, and Economical Aspects. Adv. Biochem. Eng. Biotechnol. 2005, 96, 49–125. [Google Scholar] [PubMed]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Martins, J. EPA but not DHA appears to be responsible for the efficacy of omega-3 LC-PUFA supplementation in depression: Evidence from an updated meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Dudognon, T.; Lambert, C.; Quere, C.; Auffret, M.; Soudant, P.; Kraffe, E. Mitochondrial activity, hemocyte parameters and lipid composition modulation by dietary conditioning in the Pacific oyster Crassostrea gigas. J. Comp. Physiol. B 2014, 184, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.-L.E.; Webb, K.L.; Chen, J. Seasonal changes of lipids and fatty acids in oyster tissues (Crassostrea virginica) and estuarine particulate matter. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1990, 95, 385–391. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Masci, M.; Nevigato, T.; Casini, I.; Caproni, R.; Gambelli, L.; Pellizzato, M. Growth, nutritional quality and safety of oysters (Crassostrea gigas) cultured in the lagoon of Venice (Italy). J. Sci. Food Agric. 2004, 84, 1929–1938. [Google Scholar] [CrossRef]
- Pogoda, B.; Buck, B.H.; Saborowski, R.; Hagen, W. Biochemical and elemental composition of the offshore-cultivated oysters Ostrea edulis and Crassostrea gigas. Aquaculture 2013, 400–401, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Linehan, L.G.; O'Connor, T.P.; Burnell, G. Seasonal variation in the chemical composition and fatty acid profile of Pacific oysters (Crassostrea gigas). Food Chem. 1999, 64, 211–214. [Google Scholar] [CrossRef]
- Pazos, A.J.; Ruiz, C.; Garcia-Martin, O.; Abad, M.; Sanchez, J.L. Seasonal variations of the lipid content and fatty acid composition of Crassostrea gigas cultured in El Grove, Galicia, N.W. Spain. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 114, 171–179. [Google Scholar] [CrossRef]
- Saito, H.; Marty, Y. High levels of icosapentaenoic acid in the lipids of oyster Crassostrea gigas ranging over both Japan and France. J. Oleo Sci. 2010, 59, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Soudant, P.; Van Ryckeghem, K.; Marty, Y.; Moal, J.; Samain, J.-F.; Sorgeloos, P. Comparison of the lipid class and fatty acid composition between a reproductive cycle in nature and a standard hatchery conditioning of the Pacific Oyster Crassostrea gigas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 123, 209–222. [Google Scholar] [CrossRef]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Vaz, F.M. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. 2008, 65, 2493–2506. [Google Scholar] [CrossRef] [PubMed]
- Kraffe, E.; Soudant, P.; Marty, Y.; Kervarec, N.; Jehan, P. Evidence of a tetradocosahexaenoic cardiolipin in some marine bivalves. Lipids 2002, 37, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Kariotoglou, D.M.; Mastronicolis, S.K. Sphingophosphonolipid molecular species from edible mollusks and a jellyfish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 27–44. [Google Scholar] [CrossRef]
- Le Grand, F.; Kraffe, E.; Marty, Y.; Donaghy, L.; Soudant, P. Membrane phospholipid composition of hemocytes in the Pacific oyster Crassostrea gigas and the Manila clam Ruditapes philippinarum. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T. The structure and distribution of ceramide aminoethylphosphonates in the oyster (Ostrea gigas). Biochim. Biophys. Acta 1975, 388, 353–360. [Google Scholar] [PubMed]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Dutertre, M.; Beninger, P.G.; Barillé, L.; Papin, M.; Rosa, P.; Barillé, A.-L.; Haure, J. Temperature and seston quantity and quality effects on field reproduction of farmed oysters, Crassostrea gigas, in Bourgneuf Bay, France. Aquat. Living Resour. 2009, 22, 319–329. [Google Scholar] [CrossRef]
- Lira, G.M.; Pascoal, J.C.M.; Torres, E.A.F.; Soares, R.A.M.; Mendonça, S.; Sampaio, G.R.; Correia, M.S.; Cabral, C.C.V.Q.; Cabral Júnior, C.R.; López, A.M.Q. Influence of seasonality on the chemical composition of oysters (Crassostrea rhizophorae). Food Chem. 2013, 138, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, M.A.; Racotta, I.S.; Arcos, F.; Morales-Bojórquez, E.; Moal, J.; Soudant, P.; Palacios, E. Seasonal variations of biochemical, pigment, fatty acid, and sterol compositions in female Crassostrea corteziensis oysters in relation to the reproductive cycle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kraffe, E.; Soudant, P.; Marty, Y. Fatty acids of serine, ethanolamine, and choline plasmalogens in some marine bivalves. Lipids 2004, 39, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Ruiz, C.; Martinez, D.; Mosquera, G.; Sánchez, J. Seasonal variations of lipid classes and fatty acids in flat oyster, Ostrea edulis, from San Cibran (Galicia, Spain). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995, 110, 109–118. [Google Scholar] [CrossRef]
- Brites, P.; Waterham, H.R.; Wanders, R.J.A. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2004, 1636, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, T. The role of plasmalogen in the oxidative stability of neutral lipids and phospholipids. J. Agric. Food Chem. 2010, 58, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Berenberg, C.J.; Patterson, G.W. The relationship between dietary phytosterols and the sterols of wild and cultivated oysters. Lipids 1981, 16, 276–278. [Google Scholar] [CrossRef]
- Withers, N.W.; Kokke, W.; Fenical, W.; Djerassi, C. Sterol patterns of cultured zooxanthellae isolated from marine invertebrates: Synthesis of gorgosterol and 23-desmethylgorgosterol by aposymbiotic algae. Proc. Natl. Acad. Sci. USA 1982, 79, 3764–3768. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Gary, J.J.; de Leeuw, J.W.; Johns, R.B. The fatty acid and sterol composition of two marine dinoflagellates. Phytochemistry 1984, 23, 1043–1047. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Dunstan, G.A.; Jeffrey, S.W. Geochemical significance of the occurrence of dinosterol and other 4-methyl sterols in a marine diatom. Org. Geochem. 1993, 20, 7–15. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Czarnecki, S.K.; Kyle, D.J. Effects of 4-methylsterols from algae and of β-sitosterol on cholesterol metabolism in rats. Nutr. Res. 1999, 19, 1649–1654. [Google Scholar] [CrossRef]
- Brufau, G.; Canela, M.A.; Rafecas, M. Phytosterols: Physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 2008, 28, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Do, H.Q.; Van Landeghem, L.; Wielgosz-Collin, G.; Takoudju, M.; Huvelin, J.-M.; Kornprobst, J.-M.; Bard, J.-M.; Barnathan, G.; Nazih, H. Unusual sterolic mixture, and 24-isopropylcholesterol, from the sponge Ciocalypta sp. reduce cholesterol uptake and basolateral secretion in Caco-2 cells. J. Cell. Biochem. 2009, 106, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Patch, C.; Tapsell, L.C.; Williams, P.G.; Gordon, M. Plant sterols as dietary adjuvants in the reduction of cardiovascular risk: Theory and evidence. Vasc. Health Risk Manag. 2006, 2, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kendel, M.; Couzinet-Mossion, A.; Viau, M.; Fleurence, J.; Barnathan, G.; Wielgosz-Collin, G. Seasonal composition of lipids, fatty acids, and sterols in the edible red alga Grateloupia turuturu. J. Appl. Phycol. 2013, 25, 425–432. [Google Scholar] [CrossRef]
- Beninger, P.G.; Boldina, I. Error bars in the environmental sciences: What to use, why and when. 2016; unpublished work. [Google Scholar]
- List, G.R. Soybean Lecithin: Food, Industrial Uses, and Other Applications. In Polar Lipids, Biology, Chemistry, and Technology, 1st ed.; Ahmad, M.U., Xu, X., Eds.; AOCS Press: Urbana, IL, USA, 2015; pp. 1–34. [Google Scholar]
- Fukunaga, K.; Hossain, Z.; Takahashi, K. Marine phosphatidylcholine suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by inducing apoptosis. Nutr. Res. 2008, 28, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Dupont, P. Traitement du psoriasis par la lécithine marine. Phytothérapie 2006, 4, 15–22. [Google Scholar] [CrossRef]
- Wolters, M. Diet and psoriasis: Experimental data and clinical evidence. Br. J. Dermatol. 2005, 153, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Canty, D.J.; Zeisel, S.H. Lecithin and choline in human health and disease. Nutr. Rev. 1994, 52, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Kraffe, E.; Soudant, P.; Marty, Y.; Kervarec, N. Docosahexaenoic acid-and eicosapentaenoic acid-enriched cardiolipin in the Manila clam Ruditapes philippinarum. Lipids 2005, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Jing, K.; Wu, T.; Kyu, L. Omega-3 polyunsaturated fatty acids and cancer. Anticancer Agents Med. Chem. 2013, 13, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Galli, C. n-3 Fatty acids and diabetes. Biomed. Pharmacother. 2002, 56, 397–406. [Google Scholar] [CrossRef]
- Gil, A. Polyunsaturated fatty acids and inflammatory diseases. Biomed. Pharmacother. 2002, 56, 388–396. [Google Scholar] [CrossRef]
| Collection Season | Total Lipids (% DW) | Neutral Lipids (% TL) | Glycolipids (% TL) | Phospholipids (% TL) |
|---|---|---|---|---|
| Winter (January) | 7.1 ± 0.5 | 40 ± 1 | 9 ± 2 | 50.4 ± 0.4 |
| Spring (April) | 8.6 ± 0.2 | 64.5 ± 0.7 | 7.4 ± 0.7 | 28.1 ± 0.9 |
| Summer (July) | 7.9 ± 0.1 | 39.3 ± 0.4 | 12.4 ± 0.4 | 48.0 ± 0.5 |
| Autumn (November) | 8.1 ± 0.4 | 50.3 ± 0.9 | 10.4 ± 0.8 | 39.3 ± 0.4 |
| Phospholipid Class | Winter | Spring | Summer | Autumn |
|---|---|---|---|---|
| Cardiolipin | 6.8 ± 0.3 | 10.7 ± 0.3 | 7.2 ± 0.6 | 4.9 ± 0.5 |
| Phosphatidylethanolamine | 4.3 ± 0.2 | 18 ± 2 | 6.3 ± 0.3 | 5.3 ± 0.3 |
| Ceramide aminoethylphosphonate | 10 ± 1 | 24 ± 1 | 24.6 ± 0.4 | 22 ± 1 |
| Phosphatidylserine | 1.6 ± 0.9 | 4.0 ± 0.1 | 3.2 ± 0.4 | 5.7 ± 0.2 |
| Phosphatidylcholine | 74 ± 4 | 39.7 ± 0.4 | 53.2 ± 0.3 | 58.6 ± 0.6 |
| Lysophosphatidylcholine | 2.9 ± 0.3 | 3.8 ± 0.9 | 5.4 ± 0.5 | 3.3 ± 0.5 |
| Fatty Acids (Symbol) | ECL a | Abundance (wt %) | |||
|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | ||
| Saturated Fatty Acids (SFAs) | |||||
| 14:0 | 14.00 | 1.05 ± 0.01 | 2.1 ± 0.1 | 1.46 ± 0.01 | 1.75 ± 0.04 |
| 4,8,12-Me3-13:0 | 14.49 | 0.3 ± 0.1 | 2.2 ± 0.1 | 1.73 ± 0.04 | 1.06 ± 0.04 |
| 15:0 | 15.00 | 1.2 ± 0.1 | 0.56 ± 0.09 | 0.61 ± 0.01 | 1.28 ± 0.01 |
| i-16:0 | 15.60 | 0.5 ± 0.1 | * | 0.52 ± 0.02 | 0.44 ± 0.01 |
| 16:0 | 16.00 | 14.6 ± 0.5 | 16.8 ± 0.06 | 23.8 ± 0.1 | 19.1 ± 0.3 |
| i-17:0 | 16.64 | 1.08 ± 0.01 | 0.5 ± 0.03 | 1.24 ± 0.03 | 0.84 ± 0.01 |
| 17:0 | 17.00 | 2.8 ± 0.4 | 1.41 ± 0.01 | 1.64 ± 0.03 | 2.4 ± 0.1 |
| 2-OH-16:0 | 17.18 | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.9 ± 0.03 | 1.99 ± 0.07 |
| i-18:0 | 17.64 | 0.36 ± 0.01 | 0.31 ± 0.01 | 0.88 ± 0.01 | 0.57 ± 0.02 |
| 18:0 | 18.00 | 5 ± 0.1 | 5.6 ± 0.2 | 7.32 ± 0.03 | 5.8 ± 0.2 |
| Total SFAs | 28.5 ± 0.7 | 31.2 ± 0.4 | 40.1 ± 0.1 | 35.2 ± 0.7 | |
| Monounsaturated fatty acids (MUFAs) | |||||
| 9-16:1 | 15.74 | 1.75 ± 0.01 | 1.9 ± 0.1 | 1.18 ± 0.02 | 3.16 ± 0.01 |
| 7-Me-6(Z)-16:1 | 16.20 | 0.32 ± 0.01 | n.d. | n.d. | 0.20 ± 0.01 |
| 7-Me-6(E)-16:1 | 16.53 | 0.65 ± 0.01 | 0.22 ± 0.01 | 0.36 ± 0.03 | 0.42 ± 0.02 |
| 9-18:1 | 17.76 | 2.8 ± 0.1 | 0.94 ± 0.05 | 4.78 ± 0.04 | 2.41 ± 0.07 |
| 11-18:1 | 17.81 | 4.5 ± 0.1 | 5.8 ± 0.2 | 2.46 ± 0.03 | 5.72 ± 0.06 |
| 3-19:1 | 18.58 | 1.6 ± 0.1 | 1.53 ± 0.05 | 1.36 ± 0.01 | 2.5 ± 0.01 |
| 11-20:1 | 19.68 | 1.6 ± 0.1 | 3.34 ± 0.07 | 7.19 ± 0.03 | 2.94 ± 0.03 |
| 13-20:1 | 19.73 | 5.8 ± 0.2 | 5.43 ± 0.05 | 2.16 ± 0.01 | 5.8 ± 0.1 |
| Total MUFAs | 19.0 ± 0.5 | 19.2 ± 0.6 | 19.5 ± 0.05 | 23.2 ± 0.3 | |
| Polyunsaturated fatty acids (PUFAs) | |||||
| 18:4n-3 | 17.54 | 1.01 ± 0.01 | 0.9 ± 0.02 | 1.00 ± 0.01 | 0.9 ± 0.01 |
| 18:2n-6 | 17.66 | 0.3 ± 0.1 | 0.84 ± 0.04 | 2.84 ± 0.05 | 0.67 ± 0.01 |
| 20:4n-6 | 19.24 | 4.2 ± 0.1 | 2.07 ± 0.01 | 3.35 ± 0.04 | 3.67 ± 0.01 |
| 20:5n-3 | 19.34 | 9.3 ± 0.1 | 14.5 ± 0.1 | 7.53 ± 0.03 | 9.54 ± 0.01 |
| 20:3n-7 | 19.49 | 0.5 ± 0.01 | 0.73 ± 0.03 | 0.40 ± 0.01 | 0.45 ± 0.01 |
| 20:2n-9,12 | 19.52 | 0.9 ± 0.1 | 1.28 ± 0.04 | 1.10 ± 0.01 | 1.06 ± 0.05 |
| 22:6n-3 | 21.12 | 7.8 ± 0.2 | 5.51 ± 0.09 | 9.50 ± 0.03 | 7.72 ± 0.09 |
| 22:4n-6 | 21.19 | 1.42 ±0.01 | 1.4 ± 0.05 | 2.36 ± 0.01 | 1.17 ± 0.06 |
| 22:5n-3 | 21.28 | 0.38 ± 0.01 | 0.30 ± 0.01 | 0.45 ± 0.01 | 0.37 ± 0.01 |
| 22:2n-9,15 | 21.40 | 1.31 ± 0.01 | 0.99 ± 0.01 | 1.98 ± 0.02 | 1.1 ± 0.01 |
| 22:2n-7,15 | 21.46 | 9.6 ± 0.3 | 9.02 ± 0.13 | 4.74 ± 0.05 | 7.4 ± 0.2 |
| Total PUFAs | 36.7 ± 0.7 | 37.5 ± 0.5 | 35.2 ± 0.3 | 34.1 ± 0.6 | |
| Fatty aldehyde dimethylacetals (DMAs) | |||||
| 16:0 | 16.48 | 0.68 ± 0.01 | 0.56 ± 0.08 | 0.25 ± 0.01 | 0.39 ± 0.02 |
| br-17:0 | 17.12 | 0.8 ± 0.1 | 0.39 ± 0.04 | 0.24 ± 0.01 | 0.28 ± 0.04 |
| br-17:0 | 17.22 | 0.25 ± 0.01 | * | 0.27 ± 0.01 | 0.2 ± 0.01 |
| 17:0 | 17.48 | 0.83 ± 0.01 | 0.59 ± 0.08 | * | 0.49 ± 0.07 |
| br-18:0 | 18.10 | 0.9 ± 0.1 | n.d. | n.d. | n.d. |
| br-18:0 | 18.22 | 0.82 ± 0.01 | 0.39 ± 0.02 | n.d. | 0.38 ± 0.03 |
| 18:0 | 18.48 | 6.6 ± 0.2 | 6 ± 2 | 1.49 ± 0.02 | 2.97 ± 0.03 |
| br-19:0 | 19.22 | 0.33 ± 0.01 | 0.19 ± 0.01 | * | 0.26 ± 0.02 |
| br-20:1 | 20.06 | 1 ± 0.1 | 0.42 ± 0.06 | * | 0.28 ± 0.06 |
| br-20:1 | 20.10 | 0.41 ± 0.01 | 0.37 ± 0.08 | 0.49 ± 0.01 | 0.59 ± 0.09 |
| 20:0 | 20.17 | 1.9 ± 0.2 | 1.66 ± 0.04 | 0.65 ± 0.02 | 0.63 ± 0.03 |
| Total DMAs | 14.5 ± 0.4 | 10.6 ± 0.2 | 3.39 ± 0.05 | 6.5 ± 0.3 | |
| Species | EPA % TL FA | DHA % TL FA | NMI % TL FA | Country | References |
|---|---|---|---|---|---|
| C. gigas | 10.8–15.2 | 10.3–15.5 | - | Ireland | [21] |
| C. gigas | 16.4–25.5 | 15.6–21.3 | 3.9–7.7 | Germany | [20] |
| C. rhizophorae | 17.9–19.7 | 19.7–35.8 | - | Brazil | [33] |
| Species | % PL FA | % PL FA | % PL FA | Country | References |
| C. gigas | 7.5–15.4 | 5.5–9.5 | 6.8–10.9 | France | Present study |
| C. gigas | 13.8–22.4 | 9.9–17.6 | 3.3–7.3 | Spain | [22] |
| C. gigas | 12.3 in muscle | 17.3 in muscle | 4.1 in muscle | France | [24] |
| C. gigas | 18.6–21.7 in PC | 13.1–15.8 in PC | 2.7–3.5 in PC | France | [23] |
| C. corteziensis | 10.3–17.4 | 22.5–32.5 | 7.8–12.8 | Mexico | [34] |
| O. edulis | 7.6–17.4 | 8.2–18.5 | 2.8–13 | Spain | [37] |
| Systematic Names | Trivial Names | % Total Lipids | |||
|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | ||
| 24-nor-Cholesta-5,22E-dien-3β-ol | 24-nor-Dehydrocholesterol | 5.2 ± 0.1 | 5.65 ± 0.1 | 3.57 ± 0.05 | 4.9 ± 0.2 |
| 24-nor-5α-Cholest-22E-en-3β-ol | 24-nor-Dehydrocholestanol | 0.51 ± 0.06 | n.d. | 0.47 ± 0.02 | 0.7 ± 0.07 |
| Cholesta-5,22Z-dien-3β-ol | 22Z-Dehydrocholesterol | 1.7 ± 0.3 | 1.92 ± 0.08 | 0.9 ± 0.1 | 1.7 ± 0.2 |
| Cholesta-5,22E-dien-3β-ol | 22E-Dehydrocholesterol | 8.94 ± 0.07 | 6.3 ± 0.2 | 5.6 ± 0.1 | 7.5 ± 0.4 |
| 5α-Cholest-22E-en-3β-ol | 22-Dehydrocholestanol | 1.2 ± 0.2 | n.d. | 0.8 ± 0.2 | 1.4 ± 0.2 |
| Cholest-5-en-3β-ol | Cholesterol | 32.1 ± 0.7 | 35.8 ± 0.4 | 21.4 ± 0.3 | 30.3 ± 0.4 |
| 5α-Cholestan-3β-ol | Cholestanol | 3.94 ± 0.08 | 6.7 ± 0.7 | 4 ± 0.1 | 4.7 ± 0.2 |
| 24-Methylcholesta-5,22E-dien-3β-ol | Brassicasterol/Crinosterol | 12.8 ± 0.7 | 12.6 ± 0.3 | 9.19 ± 0.05 | 10.7 ± 0.2 |
| 4α-Methyl-5α-cholest-7-en-3β-ol | Lophenol | 0.91 ± 0.08 | n.d. | n.d. | n.d. |
| X1 (Δ° C28:0) | -- | 0.5 ± 0.2 | n.d. | 1.71 ± 0.05 | n.d. |
| 24-Methylcholesta-5,24(28)-dien-3β-ol | 24-Methylenecholesterol | 14 ± 0.2 | 12.9 ± 0.5 | 8.35 ± 0.03 | 10.7 ± 0.1 |
| 24-Methylcholest-5-en-3β-ol | Campesterol/22,23-Dihydrobrassicasterol | 6.24 ± 0.07 | 6.93 ± 0.03 | 6 ± 0.1 | 5.5 ± 0.1 |
| 5α-24-Ethylcholest-25-en-3β-ol | 5α-Poriferast-25-en-3β-ol/25-Dehydroporiferastanol | 0.53 ± 0.07 | n.d. | n.d. | n.d. |
| 5α-24-Ethylcholesta-22,24(25)-dien-3β-ol | 5α-Porifera-22,24(25)-dien-3β-ol | 1.2 ± 0.1 | n.d. | 1.11 ± 0.01 | 1.1 ± 0.2 |
| 24-Ethylcholest-5,22E-dien-3β-ol | Poriferasterol/Stigmasterol | 1.78 ± 0.03 | 1.7 ± 0.2 | 1.67 ± 0.04 | 1.7 ± 0.1 |
| 4,24-Dimethylcholesta-5,7,24(28)-trien-3β-ol | 4-Methyl-5α-Ergosta-24(28)-en-3β-ol | 0.4 ± 0.03 | n.d. | 7.8 ± 0.1 | 5.6 ± 0.3 |
| 24-Ethylcholest-5-en-3β-ol | β-Sitosterol/Clionasterol | 5.4 ±0.3 | 9.9 ± 0.5 | 10.2 ± 0.2 | 6.0 ± 0.4 |
| 24-Ethyl-5α-cholest-22E-en-3β-ol | Poriferastanol/Stigmastanol | 2.8 ± 0.2 | 2.6 ± 0.2 | 1.6 ± 0.2 | 3.5 ± 0.2 |
| 24-Ethylcholesta-5,24(28)-dien-3β-ol | Fucosterol | n.d. | n.d. | 1.23 ± 0.04 | 0.65 ± 0.04 |
| 4-Methyl-24-ethyl-5α-cholesta-7-en-3β-ol | 4-Methyl-5α-Porifera-7-en-3β-ol/24-Ethyllophenol | n.d. | n.d. | 2.90 ± 0.05 | 0.96 ± 0.08 |
| X2 (Δ° C30:0) | -- | n.d. | n.d. | 0.95 ± 0.04 | n.d. |
| 4-Methyl-24-ethyl-cholesta-5-en-3β-ol | 4-Methyl-Porifera-5-en-3β-ol | n.d. | n.d. | 0.73 ± 0.09 | 0.61 ± 0.09 |
| % Phytosterols | 30.8 | 33.7 | 33.4 | 30.1 | |
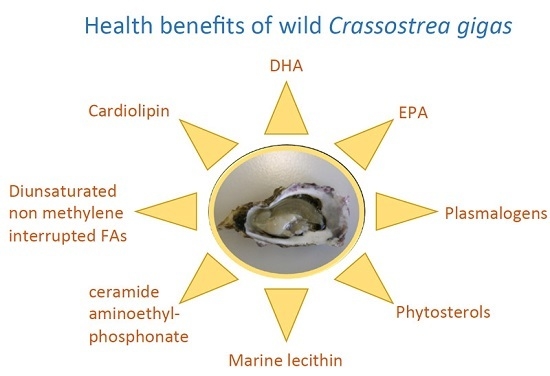
| Lipids | Potential Benefits | References |
|---|---|---|
| Lecithin (with associated PUFAs) | Protection against colon cancer | [51,54,55] |
| Treatment of psoriasis | [52] | |
| High-quality source for food industry | [49] | |
| CAEP | Implication in some haemocyte functions | [28,30] |
| Cardiolipin | Optimization of mitochondrial respiratory | [56] |
| performance | [25,26] | |
| n-3 Polyunsaturated FAs (EPA, DHA) | Improve efficacy and tolerability of cancer chemotherapy | [59] |
| Cancer prevention | [58] | |
| Neuroprotective efficacy | [60] | |
| Cardiovascular disease protection | [61] | |
| Improvement of some obesity-associated metabolic syndrome features (Type 2 diabetes) | [62,63] | |
| Anti-inflammatory effect | [64] | |
| Diunsaturated NMI FAs | Resistance to oxidative stress and microbial lipases | [38,57] |
| Plasmalogens | Countering oxidative stress | [38,39] |
| Phytosterols | Cholesterol-lowering action | [45,46] |
| Reduction in cardiovascular risk | [47] | |
| Anti-inflammatory effect | [45] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagorn, F.; Couzinet-Mossion, A.; Kendel, M.; Beninger, P.G.; Rabesaotra, V.; Barnathan, G.; Wielgosz-Collin, G. Exploitable Lipids and Fatty Acids in the Invasive Oyster Crassostrea gigas on the French Atlantic Coast. Mar. Drugs 2016, 14, 104. https://doi.org/10.3390/md14060104
Dagorn F, Couzinet-Mossion A, Kendel M, Beninger PG, Rabesaotra V, Barnathan G, Wielgosz-Collin G. Exploitable Lipids and Fatty Acids in the Invasive Oyster Crassostrea gigas on the French Atlantic Coast. Marine Drugs. 2016; 14(6):104. https://doi.org/10.3390/md14060104
Chicago/Turabian StyleDagorn, Flore, Aurélie Couzinet-Mossion, Melha Kendel, Peter G. Beninger, Vony Rabesaotra, Gilles Barnathan, and Gaëtane Wielgosz-Collin. 2016. "Exploitable Lipids and Fatty Acids in the Invasive Oyster Crassostrea gigas on the French Atlantic Coast" Marine Drugs 14, no. 6: 104. https://doi.org/10.3390/md14060104
APA StyleDagorn, F., Couzinet-Mossion, A., Kendel, M., Beninger, P. G., Rabesaotra, V., Barnathan, G., & Wielgosz-Collin, G. (2016). Exploitable Lipids and Fatty Acids in the Invasive Oyster Crassostrea gigas on the French Atlantic Coast. Marine Drugs, 14(6), 104. https://doi.org/10.3390/md14060104