Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction
Abstract
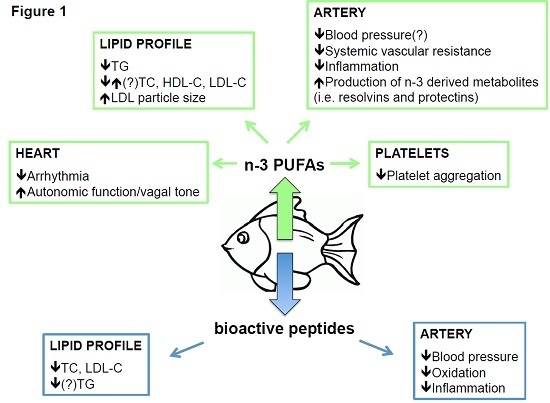
:1. Introduction
2. n-3 PUFAs and Cardiovascular Risk Factors
2.1. n-3 PUFAs and Dyslipidemias
2.2. n-3 PUFAs and Arrhythmia
2.3. n-3 PUFAs and Platelet Activity
2.4. n-3 PUFAs and Endothelial Function and Inflammation
2.5. n-3 PUFAs and Blood Pressure
3. Clinical Trials with n-3 PUFAs: Past and Future
4. Fish Proteins and Cardiovascular Risk Factors
4.1. Fish Proteins and Dyslipidemias
4.2. Fish Proteins and Hypertension
4.3. Fish Proteins and Other Potential Anti-Atherosclerotic Effects
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Voelter-Mahlknecht, S. Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin. Epigenetics 2016, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Topper, J.N.; Nagel, T.; Anderson, K.R.; Garcia-Cardena, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals N. Y. Acad. Sci. 2000, 902, 230–240. [Google Scholar] [CrossRef]
- Campbell, K.A.; Lipinski, M.J.; Doran, A.C.; Skaflen, M.D.; Fuster, V.; McNamara, C.A. Lymphocytes and the adventitial immune response in atherosclerosis. Circ. Res. 2012, 110, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Rosin, S.; Ojansivu, I.; Kopu, A.; Keto-Tokoi, M.; Gylling, H. Optimal Use of Plant Stanol Ester in the Management of Hypercholesterolemia. Cholesterol 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A., Sr.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [PubMed]
- Chiesa, G.; Parolini, C.; Sirtori, C.R. Acute effects of high-density lipoproteins: Biochemical basis and clinical findings. Curr. Opin. Cardiol. 2008, 23, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Marchesi, M.; Chiesa, G. HDL therapy for the treatment of cardiovascular diseases. Curr. Vasc. Pharmacol. 2009, 7, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; de Caterina, R. Nutraceuticals and prevention of atherosclerosis: Focus on omega-3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomistek, A.K.; Manson, J.E.; Stefanick, M.L.; Lu, B.; Sands-Lincoln, M.; Going, S.B.; Garcia, L.; Allison, M.A.; Sims, S.T.; LaMonte, M.J.; et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: Results from the Women’s Health Initiative. J. Am. Coll. Cardiol. 2013, 61, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [PubMed]
- Bang, H.O.; Dyerberg, J.; Nielsen, A.B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971, 1, 1143–1145. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Hjoorne, N. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976, 200, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [PubMed]
- Albert, C.M.; Hennekens, C.H.; O’Donnell, C.J.; Ajani, U.A.; Carey, V.J.; Willett, W.C.; Ruskin, J.N.; Manson, J.E. Fish consumption and risk of sudden cardiac death. JAMA 1998, 279, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. Omega-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Are n-3 fatty acids still cardioprotective? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Botella-Carretero, J.I.; Corella, D.; Fiol, M.; Lage, M.; Lurbe, E.; Richart, C.; Fernandez-Real, J.M.; Fuentes, F.; Ordonez, A.; et al. White fish reduces cardiovascular risk factors in patients with metabolic syndrome: The WISH-CARE study, a multicenter randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; Udenigwe, C.C. Mechanisms and prospects of food protein hydrolysates and peptide-induced hypolipidaemia. Food Funct. 2013, 4, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J. Effects of n-3 fatty acids on lipid metabolism. Annu. Rev. Nutr. 1990, 10, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. N-3 fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar] [PubMed]
- Roche, H.M.; Gibney, M.J. Effect of long-chain n-3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am. J. Clin. Nutr. 2000, 71, 232S–237S. [Google Scholar] [PubMed]
- Kang, J.X.; Leaf, A. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation 1996, 94, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Leaf, A.; Weber, P.C. Cardiovascular effects of n-3 fatty acids. N. Engl. J. Med. 1988, 318, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Hankinson, S.E.; Hotamisligil, G.S.; Rifai, N.; Willett, W.C.; Rimm, E.B. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003, 108, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.R.; Dixon, L.J.; Hanratty, C.G.; El-Sherbeeny, N.; Hamilton, P.B.; McGrath, L.T.; Leahey, W.J.; Johnston, G.D.; McVeigh, G.E. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am. J. Cardiol. 2006, 97, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, H.; Shimokawa, H.; Tagawa, T.; Kuroiwa-Matsumoto, M.; Hirooka, Y.; Takeshita, A. Long-term treatment with eicosapentaenoic acid augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilatation in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 1999, 33, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Hokanson, J.E.; Edwards, K.L. Hypertriglyceridemia as a cardiovascular risk factor. Am. J. Cardiol. 1998, 81, 7B–12B. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information. Available online: http://us.gsk.com/products/assets/us_lovaza.pdf (accessed on 20 May 2014).
- Nicholson, T.; Khademi, H.; Moghadasian, M.H. The role of marine n-3 fatty acids in improving cardiovascular health: A review. Food Funct. 2013, 4, 357–365. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.M.; Sica, D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy 2007, 27, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information. Available online: www.azpicentral.com/epanova/epanova.pdf (accessed on 31 May 2014).
- Kastelein, J.J.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Davidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova® compared to Lovaza® in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Sullivan, D.R.; Reeve, J.; Thompson, G.R. Triglyceride—Lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis 1985, 5, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish oil-how does it reduce plasma triglycerides? Biochim. Biophys. Acta 2012, 1821, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Fan, C.; Jiang, J.; Zhu, H.; Jiao, H.; Meng, Q.; Deckelbaum, R.J. Omega-3 fatty acid containing diets decrease plasma triglyceride concentrations in mice by reducing endogenous triglyceride synthesis and enhancing the blood clearance of triglyceride-rich particles. Clin. Nutr. 2008, 27, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Mangeney, M.; Cardot, P.; Loriette, C.; Rayssiguier, Y.; Chambaz, J.; Bereziat, G. Effect of dietary fish oil and corn oil on lipid metabolism and apolipoprotein gene expression by rat liver. Eur. J. Biochem. 1991, 196, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Andreo, U.; Elkind, J.; Blachford, C.; Cederbaum, A.I.; Fisher, E.A. Role of superoxide radical anion in the mechanism of apoB100 degradation induced by DHA in hepatic cells. FASEB J. 2011, 25, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Skorve, J.; Hexeberg, S.; Rustan, A.C.; Berge, R.K. The hypotriglyceridemic effect of eicosapentaenoic acid in rats is reflected in increased mitochondrial fatty acid oxidation followed by diminished lipogenesis. Lipids 1993, 28, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Cha, J.Y.; Yanagita, T.; Nakatani, N.; Oogami, K.; Imaizumi, K.; Yazawa, K. Effects of dietary alpha-linolenic, eicosapentaenoic and docosahexaenoic acids on hepatic lipogenesis and beta-oxidation in rats. Biosci. Biotechnol. Biochem. 1998, 62, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Bordin, P.; Bodamer, O.A.; Venkatesan, S.; Gray, R.M.; Bannister, P.A.; Halliday, D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur. J. Clin. Nutr. 1998, 52, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.F.; Chan, D.C.; Ooi, E.M.; Nestel, P.J.; Beilin, L.J.; Barrett, P.H. Fish oils, phytosterols and weight loss in the regulation of lipoprotein transport in the metabolic syndrome: Lessons from stable isotope tracer studies. Clin. Exp. Pharmacol. Physiol. 2006, 33, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. Fatty acid regulation of hepatic lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Harris, W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003, 44, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Swahn, E.; von Schenck, H.; Olsson, A.G. Omega-3 Ethyl Ester Concentrate Decreases Total Apolipoprotein CIII and Increases Antithrombin III in Postmyocardial Infarction Patients. Clin. Drug Investig. 1998, 15, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Minihane, A.M.; Talmud, P.J.; Wright, J.W.; Murphy, M.C.; Williams, C.M.; Griffin, B.A. Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J. Lipid Res. 2002, 43, 979–985. [Google Scholar] [PubMed]
- Harris, W.S. Fish oils and plasma lipid and lipoprotein metabolism in humans: A critical review. J. Lipid Res. 1989, 30, 785–807. [Google Scholar] [PubMed]
- Mori, T.A.; Woodman, R.J. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H. (n-3) fatty acids and cardiovascular health: Are effects of EPA and DHA shared or complementary? J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Press Release. Available online: www.vascepa.com/vascepa-pi-ppi-(clean)-P00120G-6-15.pdf (accessed on 16 October 2013).
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Ballantyne, C.M.; Braeckman, R.A.; Stirtan, W.G.; Doyle, R.T., Jr.; Philip, S.; Soni, P.N.; Juliano, R.A. Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester): Effects Upon High-Sensitivity C-Reactive Protein and Lipid Parameters in Patients With Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, H.S. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad. Med. 2014, 126, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.S.; Iaizzo, P.A.; Xiao, Y.F. Electrophysiological mechanisms of the anti-arrhythmic effects of omega-3 fatty acids. J. Cardiovasc. Trans. Res. 2011, 4, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A.; Kang, J.X.; Xiao, Y.F.; Billman, G.E. Dietary n-3 fatty acids in the prevention of cardiac arrhythmias. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 225–228. [Google Scholar] [CrossRef] [PubMed]
- London, B.; Albert, C.; Anderson, M.E.; Giles, W.R.; van Wagoner, D.R.; Balk, E.; Billman, G.E.; Chung, M.; Lands, W.; Leaf, A.; et al. Omega-3 fatty acids and cardiac arrhythmias: Prior studies and recommendations for future research: A report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation 2007, 116, e320–e335. [Google Scholar] [PubMed]
- Li, G.R.; Sun, H.Y.; Zhang, X.H.; Cheng, L.C.; Chiu, S.W.; Tse, H.F.; Lau, C.P. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+ currents and Na+ current in human atrial myocytes. Cardiovasc. Res. 2009, 81, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Soma, M.R.; Donetti, E.; Parolini, C.; Barberi, L.; Paoletti, R.; Fumagalli, R.; Catapano, A.L. Effect of lacidipine on the carotid intimal hyperplasia induced by cuff injury. J. Cardiovasc. Pharmacol. 1994, 23, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Grossfield, A.; Feller, S.E.; Pitman, M.C. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. USA 2006, 103, 4888–4893. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Kang, J.X.; Leaf, A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids 1997, 32, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Tsuburaya, R.; Yasuda, S.; Ito, Y.; Shiroto, T.; Gao, J.Y.; Ito, K.; Shimokawa, H. Eicosapentaenoic acid reduces ischemic ventricular fibrillation via altering monophasic action potential in pigs. J. Mol. Cell. Cardiol. 2011, 51, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Schmidt, E.B. Autonomic nervous system, heart rate variability and n-3 fatty acids. J. Cardiovasc. Med. 2007, 8, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Raghunathan, T.E.; King, I.; Weinmann, S.; Wicklund, K.G.; Albright, J.; Bovbjerg, V.; Arbogast, P.; Smith, H.; Kushi, L.H.; et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995, 274, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Villa, B.; Calabresi, L.; Chiesa, G.; Rise, P.; Galli, C.; Sirtori, C.R. Omega-3 fatty acid ethyl esters increase heart rate variability in patients with coronary disease. Pharmacol. Res. 2002, 45, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sutherland, F.; Rosso, R.; Teh, A.W.; Lee, G.; Heck, P.M.; Feldman, A.; Medi, C.; Watt, S.; Garg, M.L.; et al. Effects of chronic omega-3 polyunsaturated fatty acid supplementation on human atrial electrophysiology. Heart Rythm 2011, 8, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Swann, P.G.; Venton, D.L.; Le Breton, G.C. Eicosapentaenoic acid and docosahexaenoic acid are antagonists at the thromboxane A2/prostaglandin H2 receptor in human platelets. FEBS Lett. 1989, 243, 244–246. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Iversen, A.M.; Schmidt, E.B. n-3 polyunsaturated fatty acids and coronary thrombosis. Lipids 2001, 36, S79–S82. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. Omega-3 Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. BioMed Res. Int. 2015, 2015, 791978. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.P.; Nichols, B.J. Caveolae: One Function or Many? Trends Cell Biol. 2016, 26, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Layne, J.; Majkova, Z.; Smart, E.J.; Toborek, M.; Hennig, B. Caveolae: A regulatory platform for nutritional modulation of inflammatory diseases. J. Nutr. Biochem. 2011, 22, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, J.; Pastore, M.B.; Magness, R.R. Endothelial caveolar subcellular domain regulation of endothelial nitric oxide synthase. Clin. Exp. Pharmacol. Physiol. 2013, 40, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Feron, O.; Dessy, C.; Moniotte, S.; Desager, J.P.; Balligand, J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999, 103, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.L.; Stice, J.P.; Hart, C.M.; Mbai, F.N.; Knowlton, A.A. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca2+-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef]
- Chen, W.; Jump, D.B.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Chen, C.J.; Lee, T.S.; Chao, H.Y.; Wu, W.H.; Hsieh, S.C.; Sheu, H.H.; Chiang, A.N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem. 2011, 22, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Cahill, L.E.; Mozaffarian, D. Effect of fish oil on circulating adiponectin: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2013, 98, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Laiglesia, L.M.; Martinez, J.A.; Moreno-Aliaga, M.J. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J. Physiol. Biochem. 2015, 71, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; James, K.S.; Butland, B.K.; Etherington, M.D.; O’Brien, J.R.; Jones, J.G. Effects of a fish oil supplement on serum lipids, blood pressure, bleeding time, haemostatic and rheological variables. A double blind randomised controlled trial in healthy volunteers. Atherosclerosis 1987, 63, 137–143. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A. Omega-3 fatty acids and blood pressure. Cell. Mol. Biol. 2010, 56, 83–92. [Google Scholar] [PubMed]
- Minihane, A.M.; Armah, C.K.; Miles, E.A.; Madden, J.M.; Clark, A.B.; Caslake, M.J.; Packard, C.J.; Kofler, B.M.; Lietz, G.; Curtis, P.J.; et al. Consumption of Fish Oil Providing Amounts of Eicosapentaenoic Acid and Docosahexaenoic Acid That Can Be Obtained from the Diet Reduces Blood Pressure in Adults with Systolic Hypertension: A Retrospective Analysis. J. Nutr. 2016, 146, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Warltier, D.C.; Pleuss, J.A.; Hoffmann, R.G.; Goodfriend, T.L.; Egan, B.M. Effect of omega-3 fatty acids on the vascular response to angiotensin in normotensive men. Am. J. Cardiol. 1992, 70, 1347–1352. [Google Scholar] [CrossRef]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Bairati, I.; Roy, L.; Meyer, F. Double-blind, randomized, controlled trial of fish oil supplements in prevention of recurrence of stenosis after coronary angioplasty. Circulation 1992, 85, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Saito, Y.; Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008, 200, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [PubMed]
- Nodari, S.; Triggiani, M.; Campia, U.; Manerba, A.; Milesi, G.; Cesana, B.M.; Gheorghiade, M.; Dei Cas, L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Raitt, M.H.; Connor, W.E.; Morris, C.; Kron, J.; Halperin, B.; Chugh, S.S.; McClelland, J.; Cook, J.; MacMurdy, K.; Swenson, R.; et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: A randomized controlled trial. JAMA 2005, 293, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; Raitt, M.H.; Dullemeijer, C.; Kraemer, D.F.; Zock, P.L.; Morris, C.; Katan, M.B.; Connor, W.E.; Camm, J.A.; Schouten, E.G.; et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur. Heart J. 2009, 30, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. Alpha Omega Trial Group n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S.; SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Diaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [PubMed]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; et al. Fish oil and postoperative atrial fibrillation: The Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012, 308, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br. J. Nutr. 2012, 107, S201–S213. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E. Update on the Vitamin D and OmegA-3 trial (VITAL). J. Steroid Biochem. Mol. Biol. 2016, 155, 252–256. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Anderson, G.H. Recent advances in dietary proteins and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Tepper, S.A.; Czarnecki, S.K.; Klurfeld, D.M. Atherogenicity of animal and vegetable protein. Influence of the lysine to arginine ratio. Atherosclerosis 1982, 41, 429–431. [Google Scholar] [CrossRef]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mork, S.; Berge, R.K. Fish protein hydrolysate reduces plasma total cholesterol, increases the proportion of HDL cholesterol, and lowers acyl-CoA: Cholesterol acyltransferase activity in liver of Zucker rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [PubMed]
- Carroll, K.K.; Hamilton, R.M.G. Effects of dietary protein and carbohydrate on plasma cholesterol levels in relation to atherosclerosis. J. Food Sci. 1975, 40, 18–23. [Google Scholar] [CrossRef]
- Zhang, X.; Beynen, A.C. Influence of dietary fish proteins on plasma and liver cholesterol concentrations in rats. Br. J. Nutr. 1993, 69, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Bettzieche, A.; Hirche, F.; Brandsch, C.; Stangl, G.I.; Eder, K. Dietary fish protein alters blood lipid concentrations and hepatic genes involved in cholesterol homeostasis in the rat model. Br. J. Nutr. 2006, 96, 674–682. [Google Scholar] [PubMed]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein hydrolysates affect cholesterol metabolism in rats fed non-cholesterol and high-cholesterol diets. J. Med. Food 2012, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Vikoren, L.A.; Nygard, O.K.; Lied, E.; Rostrup, E.; Gudbrandsen, O.A. A randomised study on the effects of fish protein supplement on glucose tolerance, lipids and body composition in overweight adults. Br. J. Nutr. 2013, 109, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. Nutr. 2013, 53, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide inhibitors for angiotensin I-converting enzyme from thermolysin digest of dried bonito. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Watanabe, K.; Ma, M.; Hirayama, M.; Kobayashi, T.; Oyama, H.; Sakaguchi, Y.; Kanda, M.; Kodama, M.; Aizawa, Y. The Effects of gamma-Aminobutyric Acid, Vinegar, and Dried Bonito on Blood Pressure in Normotensive and Mildly or Moderately Hypertensive Volunteers. J. Clin. Biochem. Nutr. 2009, 45, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hu. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsui, T.; Ushida, Y.; Matsumoto, K. Vasodilating effect of di-peptides in thoracic aortas from spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 2292–2295. [Google Scholar] [CrossRef] [PubMed]
- Enari, H.; Takahashi, Y.; Kawarasaki, M.; Tada, M.; Tatsuta, K. Identification of angiotensin I-converting enzymeinhibitory peptides derived from salmon muscleand their antihypertensive effect. Fish. Sci. 2008, 74, 911–920. [Google Scholar] [CrossRef]
- Ichimura, T.; Hu, J.; Aita, D.Q.; Maruyama, S. Angiotensin I-converting enzyme inhibitory activity and insulin secretion stimulative activity of fermented fish sauce. J. Biosci. Bioeng. 2003, 96, 496–499. [Google Scholar] [CrossRef]
- Manikkam, V.; Vasiljevic, T.; Donkor, O.N.; Mathai, M.L. A Review of Potential Marine-derived Hypotensive and Anti-obesity Peptides. Crit. Rev. Food Sci. Nutr. 2016, 56, 92–112. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.; Morimura, S.; Guo, H.C.; Shigematsu, T.; Kida, K.; EUemura, Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Structure and activity of angiotensin I converting enzyme inhibitory peptides derived from Alaskan pollack skin. J. Biochem. Mol. Biol. 2002, 35, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, M.; Morita, A.; Kakitani, M.; Okada, M.; Yokogoshi, H. Inhibition of the endothelin-converting enzyme by pepsin digests of food proteins. Biosci. Biotechnol. Biochem. 1995, 59, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: A randomized controlled trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, G.; Mitchell, P.L.; Rioux, L.E.; Hasan, F.; Jin, T.; Roblet, C.R.; Doyen, A.; Pilon, G.; St-Pierre, P.; Lavigne, C.; et al. Low-Molecular-Weight Peptides from Salmon Protein Prevent Obesity-Linked Glucose Intolerance, Inflammation, and Dyslipidemia in LDLR−/−/ApoB100/100 Mice. J. Nutr. 2015, 145, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjorndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in ApoE-deficient mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Mar. Drugs 2016, 14, 113. https://doi.org/10.3390/md14060113
Chiesa G, Busnelli M, Manzini S, Parolini C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Marine Drugs. 2016; 14(6):113. https://doi.org/10.3390/md14060113
Chicago/Turabian StyleChiesa, Giulia, Marco Busnelli, Stefano Manzini, and Cinzia Parolini. 2016. "Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction" Marine Drugs 14, no. 6: 113. https://doi.org/10.3390/md14060113
APA StyleChiesa, G., Busnelli, M., Manzini, S., & Parolini, C. (2016). Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Marine Drugs, 14(6), 113. https://doi.org/10.3390/md14060113