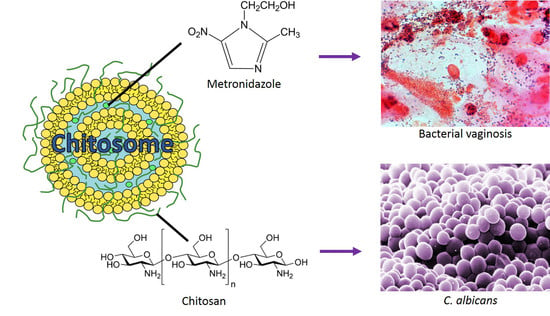
Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Vesicles
2.2. Metronidazole Entrapment Efficiency
2.3. In Vitro Release Studies
2.4. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Vesicles
3.3. Size Reduction of Vesicles
3.4. Entrapment of Metronidazole
3.5. Lipid Content
3.6. Particle Size Analysis
3.7. Determination of Zeta Potential
3.8. In Vitro Release Studies
3.9. Antifungal Activity Testing
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F. Risk factors for bacterial vaginosis. Eur. J. Obstet. Gynecol. Reproduct. Biol. 2004, 117, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P. Vulvovaginal candidiasis and bacterial vaginosis. Infect. Dis. Clin. N. Am. 2008, 22, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Mashburn, J.J. Vaginal infections update. J. Midwifery Women’s Health 2012, 57, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, B. Diagnosing vaginal infections: It’s time to join the 21st century. Curr. Infect. Dis. Rep. 2010, 12, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.R. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.; Bellen, G.; Ausma, J.; Verguts, L.; Vaneldere, J.; Hinoul, P.; Borgers, M.; Janssens, D. The effect of antifungal treatment on the vaginal flora of women with vulvo-vaginal yeast infection with or without bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Scuota, S.; Rossi, C. FG90 chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int. J. Pharm. 2009, 377, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T. Novel Chitosan-Containing Liposomes as Mucoadhesive Delivery System for Vaginal Administration. Ph.D. Thesis, University of Tromsø The Arctic University of Norway, Tromsø, Norway, 2015. [Google Scholar]
- Andersen, T.; Bleher, S.; Flaten, G.E.; Tho, I.; Mattsson, S.; Škalko-Basnet, N. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Mar. Drugs 2015, 13, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanić, Ž.; Škalko-Basnet, N. Mucosal nanosystems for improved topical drug delivery: Vaginal route of administration. J. Drug Deliv. Sci. Technol. 2014, 24, 435–444. [Google Scholar] [CrossRef]
- Li, N.; Zhuang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zaru, M.; Manca, M.-L.; Fadda, A.M.; Antimisiaris, S.G. Chitosan-coated liposomes for delivery to lungs by nebulization. Colloid Surf. B Biointerfaces 2009, 71, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Duennhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.; Harris, R.; Cordoba-Diaz, M.; Elorza, M.; Elorza, B.; Lenoir, J.; Adriaens, E.; Remon, J.P.; Heras, A.; Cordoba-Diaz, D. Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur. J. Pharm. Sci. 2013, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Suljaković, S.; Škalko-Basnet, N.; Kristl, A. Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. Eur. J. Pharm. Biopharm. 2014, 87, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Tho, I.; Vanic, Z.; Skalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan and antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.-C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucoamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [PubMed]
- Pu, Y.U.; Liu, A.; Zheng, Y.; Ye, B.I.N. In vitro damage of Candida albicans biofilms by chitosan. Exp. Ther. Med. 2014, 8, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Palmeira-de-Oliveira, A.; Miranda, I.M.; Branco, J.; Colbrado, L.; Moneteiro-Soares, M.; Queiroz, J.A.; Pina-Vaz, C.; Rodrigues, A.G. Anti-biofilm activity of low-molecular weight chitosan hydrogel against Candida species. Mol. Microbiol. Immunol. 2014, 203, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of chitosan gels to disrupt bacterial biofilms and their applications in the treatment of bacterial vaginosis. J. Pharm. Sci. 2013, 102, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, Ž.; Škalko-Basnet, N.; Schubert, R. Liposomal gels for vaginal drug delivery. Int. J. Pharm. 2001, 219, 139–149. [Google Scholar] [CrossRef]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, mucoadhesive and release properties of carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Ndesendo, V.M.K.; Pillay, V.; Choonara, Y.E.; Buchmann, E.; Bayever, D.N.; Meyer, L.C.R. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech 2008, 9, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Vanić, Ž.; Flaten, G.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Pectosomes and chitosomes as delivery systems for metronidazole: The one-pot preparation method. Pharmaceutics 2013, 5, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin. Drug Deliv. 2011, 8, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Bernkop-Schnurch, A. Strategies for improving mucosal drug delivery. Nanomedicine (Lond.) 2013, 8, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Lubrizol. Formulating controlled release tablets and capsules with carbopol polymers. In Pharmaceutical Bulletin; Lubrizol: Wickliffe, OH, USA, 2011; Volume 31. [Google Scholar]
- Hainer, B.L.; Gibson, M.V. Vaginitis: Diagnosis and Treatment. Am. Fam. Phys. 2011, 83, 807–815. [Google Scholar]
- Wain, A.M. Metronidazole vaginal gel 0.75% (MetroGel-Vagina®): A brief review. Inf. Dis. Obstet. Gynecol. 1998, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ensing, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar]
- Zupančić, Š.; Potrč, T.; Baumgartner, S.; Kocbek, P.; Kristl, J. Formulation and evaluation of chitosan/polyethylene oxide nanofibers loaded with metronidazole for local infections. Eur. J. Pharm. Sci. 2016, 95, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, M.H.; Park, S.C.; Cheong, H.; Jang, M.K.; Nah, J.W.; Hahm, K.S. Investigation of the antifungal activity and mechanism of action of lmws-chitosan. J. Microbiol. Biotechnol. 2008, 18, 1729–1734. [Google Scholar] [PubMed]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Schollmeyer, E. Anticandidal action of fungal chitosan against Candida albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Hawser, S.P.; Douglas, L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 1995, 39, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, M.V.; Garland, S.M. Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics. J. Clin. Microbiol. 2006, 44, 3213–3217. [Google Scholar] [CrossRef] [PubMed]
- Pradines, B.; Bories, C.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Drug-free chitosan coated poly(isobutylcyanoacrylate) nanoparticles are active against Trihomonas vaginalis and non-toxic towards pig vaginal mucosa. Pharm. Res. 2015, 32, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Melkumyan, A.R.; Priputnevich, T.V.; Ankirskaya, A.S.; Murav’eva, V.V.; Lubasovskaya, L.A. Effects of antibiotic treatment on the Lactobacillus composition of vaginal microbiota. Bull. Exp. Biol. Med. 2015, 158, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [PubMed]
- Sperstad, S.V.; Haug, T.; Paulsen, V.; Rode, T.M.; Strandskog, G.; Solem, S.T.; Styrvold, O.B.; Stensvåg, K. Characterization of crustins from the hemocytes of the spider crab, Hyas araneus, and the red king crab, Paralithodes camtschaticus. Dev. Comp. Immunol. 2009, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]



| Type of Liposomes (Sonication Time) | Peak 1 * | Peak 2 * | PI | ||
|---|---|---|---|---|---|
| Size (nm) | % | Size (nm) | % | ||
| Chitosan-containing (1 min) | 44.9 | 35 | 188.7 | 59 | 0.357 |
| Carbopol-containing (1 min) | 90.9 | 15 | 508.6 | 83 | 0.456 |
| Carbopol-containing (2 min) | 72.0 | 15 | 401.6 | 85 | 0.517 |
| Plain (1 min) | 41.4 | 13 | 224.5 | 86 | 0.368 |
| Type of Liposomes (Sonication Time) | Zeta Potential (mV) |
|---|---|
| Chitosan-containing (1 min) | 10.6 ± 1.3 |
| Carbopol-containing (1 min) | −4.2 ± 0.4 |
| Carbopol-containing (2 min) | −2.3 ± 0.5 |
| Plain (1 min) | −0.5 ± 0.7 |
| Formulation | C. albicans Inhibition |
|---|---|
| Chitosan (mg/mL) | |
| Chitosan-containing (MTZ) | 0.11–0.22 |
| Chitosan-containing (no drug) | 0.11–0.22 |
| Carbopol-containing (MTZ) | No inhibition |
| Plain (MTZ) | No inhibition |
| Metronidazole in solution (control) | No inhibition |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. https://doi.org/10.3390/md15030064
Andersen T, Mishchenko E, Flaten GE, Sollid JUE, Mattsson S, Tho I, Škalko-Basnet N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Marine Drugs. 2017; 15(3):64. https://doi.org/10.3390/md15030064
Chicago/Turabian StyleAndersen, Toril, Ekaterina Mishchenko, Gøril Eide Flaten, Johanna U. Ericson Sollid, Sofia Mattsson, Ingunn Tho, and Nataša Škalko-Basnet. 2017. "Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes" Marine Drugs 15, no. 3: 64. https://doi.org/10.3390/md15030064
APA StyleAndersen, T., Mishchenko, E., Flaten, G. E., Sollid, J. U. E., Mattsson, S., Tho, I., & Škalko-Basnet, N. (2017). Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Marine Drugs, 15(3), 64. https://doi.org/10.3390/md15030064