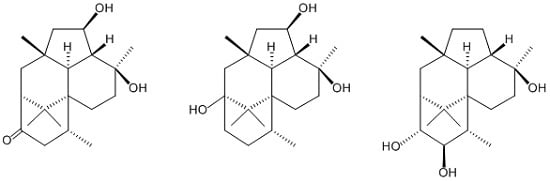
Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Culturing and Isolation of Metabolites
4.4. Formation of the (S)- and (R)-MTPA Esters of 1
4.5. Formation of the (S)- and (R)-MTPA Esters of 2
4.6. Assay for Cytotoxicity
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nicoletti, R.; Trincone, A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F. Natural products from marine fungi-still anunderrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Muroga, Y.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins I–O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron 2009, 65, 7580–7586. [Google Scholar] [CrossRef]
- Yamada, T.; Kitada, H.; Kajimoto, T.; Numata, A.; Tanaka, R. The relationship between the CD cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. J. Org. Chem. 2010, 75, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kikuchi, T.; Tanaka, R.; Numata, A. Halichoblelides B and C, potent cytotoxic macrolides from a Streptomyces species separated from a marine fish. Tetrahedron Lett. 2012, 53, 2842–2846. [Google Scholar] [CrossRef]
- Kitano, M.; Yamada, T.; Amagata, T.; Minoura, K.; Tanaka, R.; Numata, A. Novel pyridino-α-pyrone sesquiterpene type pileotin produced by a sea urchin-derived Aspergillus sp. Tetrahedron Lett. 2012, 53, 4192–4194. [Google Scholar] [CrossRef]
- Yamada, T.; Mizutani, Y.; Umebayashi, Y.; Inno, N.; Kawashima, M.; Kikuchi, T.; Tanaka, R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014, 55, 662–664. [Google Scholar] [CrossRef]
- Yamada, T.; Umebayashi, Y.; Kawashima, M.; Sugiura, Y.; Kikuchi, T.; Tanaka, R. Determination of the chemical structures of Tandyukisins B–D, isolated from a marine sponge-derived fungus. Mar. Drugs 2015, 13, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Suzue, M.; Kikuchi, T.; Tanaka, R.; Yamada, T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett. 2016, 57, 5070–5073. [Google Scholar] [CrossRef]
- Sun, P.-X.; Zheng, C.-J.; Li, W.-C.; Huang, F.; Qin, L.-P. Trichodermanin A, a novel diterpenoid from endophytic fungus culture. J. Nat. Med. 2011, 65, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Izumi, N.; Ui, H.; Sueki, A.; Masuma, R.; Nonaka, K.; Hirose, T.; Sunazuka, T.; Nagai, T.; Yamada, H.; et al. Wickerols A and B: Novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron 2012, 68, 9267–9271. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]




| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH a | δC | δH a | δC | δH a | δC | |
| 1α | - | 217.7 (s) | 1.90 ddd (14.4, 2.4, 2.4) | 35.5 (t) | - | - |
| 1β | - | - | 1.98 ddd (14.4, 10.8, 6.0) | - | 4.11 d (5.4) | 80.4 (d) |
| 2α | 2.27 dd (20.4, 7.2) | 48.7 (t) | 1.64 m | 29.5 (t) | 3.88 dd (7.8, 5.4) | 83.7 (d) |
| 2β | 2.91 dd (20.4, 9.0) | - | 2.12 m | - | - | - |
| 3 | 2.44 dqd (9.0,7.2,7.2) | 26.1 (d) | 2.14 m | 26.0 (d) | 1.88 qd (7.8, 7.8) | 36.6 (d) |
| 4 | - | 39.5 (s) | - | 41.0 (s) | - | 41.2 (s) |
| 5 | - | 38.2 (s) | - | 44.1 (s) | - | 39.4 (s) |
| 6 | 2.03 dd (3.6, 3.6) | 58.0 (d) | - | 74.9 (s) | 1.50 dd (4.8, 3.0) | 53.2 (d) |
| 7α | 1.76 dd (13.8, 3.6) | 41.4 (t) | 1.56 m | 51.2 (t) | 1.78 dd (13.8, 4.8) | 40.9 (t) |
| 7β | 1.92 dd (13.8, 3.6) | - | 1.62 m | - | 1.70 dd (13.8, 3.0) | - |
| 8 | - | 39.0 (s) | - | 39.1 (s) | - | 39.6 (s) |
| 9α | 1.50 m | 53.9 (t) | 1.51 m | 54.4 (t) | 1.03 m | 43.5 (t) |
| 9β | 1.50 m | - | 1.51 m | - | 1.43 m | - |
| 10α | 4.41 ddd (7.8, 4.8, 1.2) | 72.6 (d) | 4.39 ddd (8.4, 4.8, 1.2) | 72.8 (d) | 1.59 m | 21.6 (t) |
| 10β | - | - | - | - | 1.80 m | - |
| 11 | 1.95 dd (12.6, 4.8) | 54.7 (d) | 1.88 dd (12.6, 4.8) | 55.1 (d) | 1.81 dd (13.2, 4.2) | 44.2 (d) |
| 12 | 1.46 d (12.6) | 51.0 (d) | 1.25 d (12.6) | 50.4 (d) | 1.32 d (13.2) | 51.8 (d) |
| 13α | 1.25 ddd (14.0, 14.0, 3.0) | 25.9 (t) | 1.23 ddd (14.0, 14.0, 3.0) | 26.4 (t) | 1.23 ddd (13.8, 13.8, 3.6) | 26.3 (t) |
| 13β | 1.80 ddd (14.0, 3.0, 3.0, ) | - | 1.73 ddd (14.0, 3.0, 3.0) | - | 1.72 ddd (13.8, 3.6, 3.6) | - |
| 14α | 1.66 ddd (14.0, 3.0, 3.0) | 40.2 (t) | 1.66 m | 40.6 (t) | 1.64 ddd (13.8, 3.6, 3.6) | 41.1 (t) |
| 14β | 1.55 ddd (14.0, 14.0, 3.0) | - | 1.59 m | - | 1.46 ddd (13.8, 13.8, 3.6) | - |
| 15 | - | 72.9 (s) | - | 73.1 (s) | - | 73.6 (s) |
| 16 | 1.26 s | 21.6 (q) | 1.23 s | 21.5 (q) | 1.18 s | 20.5 (q) |
| 17 | 1.17 d (7.2) | 21.3 (q) | 1.05 d (6.6) | 22.9 (q) | 1.23 d (7.8) | 20.0 (q) |
| 18ax | 1.01 s | 24.2 (q) | 0.93 s | 18.3 (q) | 0.99 s | 25.7 (q) |
| 19eq | 1.03 s | 25.1 (q) | 1.02 s | 19.4 (q) | 1.04 s | 25.2 (q) |
| 20 | 1.05 s | 22.0 (q) | 1.29 s | 20.9 (q) | 0.98 s | 19.8 (q) |
| Compounds | Cell line P388 | Cell line HL-60 | Cell line L1210 |
|---|---|---|---|
| IC50 (µM) a | IC50 (µM) a | IC50 (µM) a | |
| 1 | 7.9 | 6.8 | 7.6 |
| 2 | 51.9 | 59.7 | 85.2 |
| 3 | 80.1 | 78.9 | 134.1 |
| 5-fluorouracil b | 6.1 | 5.1 | 4.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus. Mar. Drugs 2017, 15, 169. https://doi.org/10.3390/md15060169
Yamada T, Suzue M, Arai T, Kikuchi T, Tanaka R. Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus. Marine Drugs. 2017; 15(6):169. https://doi.org/10.3390/md15060169
Chicago/Turabian StyleYamada, Takeshi, Mayo Suzue, Takanobu Arai, Takashi Kikuchi, and Reiko Tanaka. 2017. "Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus" Marine Drugs 15, no. 6: 169. https://doi.org/10.3390/md15060169
APA StyleYamada, T., Suzue, M., Arai, T., Kikuchi, T., & Tanaka, R. (2017). Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus. Marine Drugs, 15(6), 169. https://doi.org/10.3390/md15060169