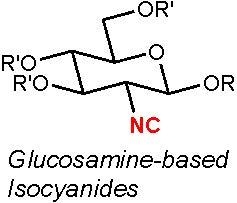
Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides
Abstract
:1. Introduction
2. Results
2.1. Compounds with Ester Groups at the C-1 Position
2.2. Compounds with Ether at the C-1 Position
2.3. Effects of Substituents at the C-3, 4, and 6 Positions
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Preparation of Formamides 7a and 7b
4.2. General Procedure for the Preparation of Isocyanides 6a–k
4.3. General Procedure for the Preparation of Glycosides 9d–f
4.4. General Procedure for the Preparation of Glycosides 9c and 9g
4.5. General Procedure for the Preparation of Formamides 7c–g from Glycosides 9c–g
4.6. Synthesis of 7h
4.7. Synthesis of 7i
4.8. Synthesis of 7j
4.9. Synthesis of 7k
4.10. Antifouling Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global Wind Energy Council. Global Wind Report Annual Market Update 2015; GWEC: Brussels, Belgium, 2015; Available online: http://www.gwec.net/wp-content/uploads/vip/GWEC-Global-Wind-2015-Report_April-2016_22_04.pdf (accessed on 20 April 2017).
- Fitridge, I.; Dempster, T.; Guenther, J.; de Nys, R. The impact and control of biofouling in marine aquaculture: A review. Biofouling 2012, 28, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Champ, M.A. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef]
- Flemming, H.-C. Reverse osmosis membrane biofouling. Exp. Therm. Fluid Sci. 1997, 14, 382–391. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Waldock, M. Copper Biocides in the Marine Environment. In Ecotoxicology of Antifouling Biocides; Arai, T., Harino, H., Ohji, M., Langston, W.J., Eds.; Springer: Tokyo, Japan, 2009; pp. 413–430. [Google Scholar]
- Horiguchi, T.; Shiraishi, H.; Shimizu, M.; Yamazaki, S.; Morita, M. Imposex in Japanese gastropods (Neogastropoda and Mesogastropoda): Effects of tributyltin and triphenyltin from antifouling paints. Mar. Pollut. Bull. 1995, 31, 402–405. [Google Scholar] [CrossRef]
- Shimasaki, Y.; Kitano, T.; Oshima, Y.; Inoue, S.; Imada, N.; Honjo, T. Tributyltin causes masculinization in fish. Environ. Toxicol. Chem. 2003, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.G.; Kime, D.E. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat. Toxicol. 2003, 65, 309–316. [Google Scholar] [CrossRef]
- Weis, J.S.; Perlmutter, J. Effects of tributyltin on activity and burrowing behavior of the fiddler crab, Uca pugilator. Estuaries 1987, 10, 342–346. [Google Scholar] [CrossRef]
- Weis, J.S.; Kim, K. Tributyltin is a teratogen in producing deformities in limbs of the fiddler crab, Uca pugilator. Arch. Environ. Contam. Toxicol. 1988, 17, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, A.; Delos, A.L.; Garaventa, F.; Faimali, M.; Gerace, S. Limited effectiveness of marine protected areas: Imposex in Hexaplex trunculus (Gastropoda, Muricidae) populations from Italian marine reserves. Mar. Pollut. Bull. 2004, 48, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M. TBT or not TBT?: That is the question. Biofouling 1999, 14, 117–129. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Bryan, G.W.J. Reproductive failure in populations of the dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from antifouling paints. Mar. Biol. Assoc. UK 1986, 66, 767–777. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Bryan, G.W. TBT-induced imposex in neogastropod snails: Masculinization to mass extinction. In Tributyltin: Case Study of an Environmental Contaminant; de Mora, S.J., Ed.; Cambridge University Press: Cambridge, UK, 1996; pp. 212–236. [Google Scholar]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Steinberg, P.D.; de Nys, R.; Kjelleberg, S. Chemical cues for surface colonization. J. Chem. Ecol. 2002, 28, 1935–1951. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, T.V.; Limna Mol, V.P. Natural product antifoulants. Curr. Sci. 2009, 97, 508–520. [Google Scholar]
- Maréchal, J.-P.; Hellio, C. Challenges for the development of new non-toxic antifouling solutions. Int. J. Mol. Sci. 2009, 10, 4623–4637. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2009, 26, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N. Antifouling marine natural products. Nat. Prod. Rep. 2011, 28, 400–410. [Google Scholar] [CrossRef] [PubMed]
- White, D.E.; Stewart, I.C.; Grubbs, R.H.; Stoltz, B.M. The catalytic asymmetric total synthesis of elatol. J. Am. Chem. Soc. 2008, 130, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Oguri, Y.; Matsuura, H.; Yamazaki, S.; Suzuki, M.; Yoshimura, E.; Furuta, T.; Nogata, Y.; Serisawa, Y.; Matsuyama-Serisawa, K.; et al. Omaezallene from red alga Laurencia sp.: Structure elucidation, total synthesis, and antifouling activity. Angew. Chem. Int. Ed. 2014, 53, 3909–3912. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling sesquiterpenes from four nudibranchs of the family Phyllidiidae. Tetrahedron 1996, 52, 9447–9454. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakahara, H.; Shirokura, Y.; Nogata, Y.; Yoshimura, E.; Umezawa, T.; Okino, T.; Matsuda, F. Total synthesis of 10-isocyano-4-cadinene and determination of and its absolute configuration. Org. Lett. 2010, 12, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Nakahara, H.; Shirokura, Y.; Nogata, Y.; Yoshimura, E.; Umezawa, T.; Okino, T.; Matsuda, F. Total synthesis of 10-isocyano-4-cadinene and its stereoisomers and evaluations of antifouling activities. J. Org. Chem. 2011, 76, 6558–6573. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Umezawa, T.; Garson, M.J.; Matsuda, F. Confirmation of the configuration of 10-isothiocyanato-4-cadinene diastereomers through synthesis. J. Nat. Prod. 2012, 75, 2232–2235. [Google Scholar] [CrossRef] [PubMed]
- Gulavita, N.K.; De Silva, E.D.; Hagadone, M.R.; Karuso, P.; Scheuer, P.J.; Van Duyne, G.D.; Clardy, J. Nitrogenous bisabolene sesquiterpenes from marine invertebrates. J. Org. Chem. 1986, 51, 5136–5139. [Google Scholar] [CrossRef]
- Kassühlke, K.E.; Potts, B.C.M.; Faulkner, D.J. New nitrogenous sesquiterpenes from two Philippine nudibranchs, Phyllidia pustulosa and P. varicosa, and from a Palauan sponge, Halichondria cf. lendenfeldi. J. Org. Chem. 1991, 56, 3747–3750. [Google Scholar] [CrossRef]
- Kitano, Y.; Ito, T.; Suzuki, T.; Nogata, Y.; Shinshima, K.; Yoshimura, E.; Chiba, K.; Tada, M.; Sakaguchi, I. Synthesis and antifouling activity of 3-isocyanotheonellin and its analogues. J. Chem. Soc. Perkin Trans. 1 2002, 2251–2255. [Google Scholar] [CrossRef]
- Nogata, Y.; Kitano, Y.; Yoshimura, E.; Shinshima, K.; Sakaguchi, I. Antifouling activity of simple synthetic isocyanides against larvae of the barnacle Balanus amphitrite. Biofouling 2004, 20, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Wagatsuma, H.; Kominami, Y.; Nogata, Y.; Yoshimura, E.; Chiba, K.; Kitano, Y. Anti-barnacle activity of isocyanides derived from amino acids. Chem. Biodivers. 2016, 13, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Grenouillat, N.; Vauzeilles, B.; Beau, J.-M. Lipid analogs of the nodulation factors using the Ugi/Passerini multicomponent reactions: Preliminary studies on the carbohydrate monomer. Heterocycles 2007, 73, 891–901. [Google Scholar]
- Tavecchia, P.; Trumtel, M.; Veyrières, A.; Sinay, P. Glycosidations with N-formylamino sugar: A new approach to 2′-deoxy-β-disaccharides. Tetrahedron Lett. 1989, 43, 2533–2536. [Google Scholar] [CrossRef]
- Kitano, Y.; Nogata, Y.; Matsumura, K.; Yoshimura, E.; Chiba, K.; Tada, M.; Sakaguchi, I. Design and synthesis of anti-barnacle active fluorescence-labeled probe compounds and direct observation of the target region in barnacle cypris larvae for dimethyl-isocyanoalkyl compounds. Tetrahedron 2005, 61, 9969–9973. [Google Scholar] [CrossRef]
- Fujiwara, S.; Akima, C.; Nogata, Y.; Yoshimura, E.; Chiba, K.; Kitano, Y. Bio-organic and anti-barnacle studies of fluorescence-labeled probe compounds against cyprids of barnacles. J. Exp. Mar. Biol. Ecol. 2013, 445, 88–92. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umezawa, T.; Hasegawa, Y.; Novita, I.S.; Suzuki, J.; Morozumi, T.; Nogata, Y.; Yoshimura, E.; Matsuda, F. Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides. Mar. Drugs 2017, 15, 203. https://doi.org/10.3390/md15070203
Umezawa T, Hasegawa Y, Novita IS, Suzuki J, Morozumi T, Nogata Y, Yoshimura E, Matsuda F. Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides. Marine Drugs. 2017; 15(7):203. https://doi.org/10.3390/md15070203
Chicago/Turabian StyleUmezawa, Taiki, Yuki Hasegawa, Ira S. Novita, Junya Suzuki, Tatsuya Morozumi, Yasuyuki Nogata, Erina Yoshimura, and Fuyuhiko Matsuda. 2017. "Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides" Marine Drugs 15, no. 7: 203. https://doi.org/10.3390/md15070203
APA StyleUmezawa, T., Hasegawa, Y., Novita, I. S., Suzuki, J., Morozumi, T., Nogata, Y., Yoshimura, E., & Matsuda, F. (2017). Design, Synthesis, and Antifouling Activity of Glucosamine-Based Isocyanides. Marine Drugs, 15(7), 203. https://doi.org/10.3390/md15070203