Antibacterial and Antioxidant Capacities and Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by Low-Molecular-Weight Fucoidans Prepared from Compressional-Puffing-Pretreated Sargassum Crassifolium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of LMW Fucoidans
2.2. Physicochemical and Compositional Analyses of Native and LMW Fucoidans
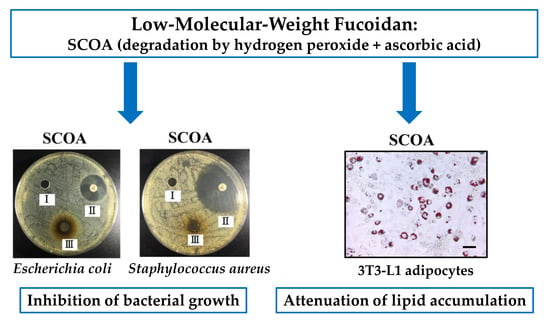
2.3. Antibacterial Activities of SC, SCO, SCA, SCOA, and SCH
2.4. Antioxidant Activities of of SC, SCO, SCA, SCOA, and SCH
2.5. Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by SC, SCO, SCA, SCOA, and SCH
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Compressional-Puffing Procedure
3.3. Water Extraction Procedure
3.4. Degradation Procedure
3.5. Chemical Methods
3.6. Analysis of Monosaccharide Composition
3.7. Intrinsic Viscosity Analysis
3.8. Agarose Gel Electrophoresis
3.9. Fourier Transform Infrared (FTIR) Spectroscopy
3.10. Molecular Weight Analysis
3.11. Zone of Inhibition
3.12. DPPH Radical Scavenging Activity
3.13. ABTS Radical Cation Scavenging Activity
3.14. FRAP Assay
3.15. Adipocyte Cell Culture
3.16. Cell Viability Test
3.17. Lipid Accumulation
3.18. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wawruch, M.; Bozekova, L.; Krcmery, S.; Kriska, M. Risks of antibiotic treatment. Bratisl. Lek. Listy 2002, 103, 270–275. [Google Scholar] [PubMed]
- Abdullah, A.S.H.; Mirghani, M.E.S.; Jamal, P. Antibacterial activity of malaysian mango kernel. Afr. J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.F.; Li, P.C. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sallmyr, A.; Fan, J.; Rassool, F.V. Genomic instability in myeloid malignancies: Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008, 270, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Branen, A.L. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975, 52, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Fukushima, S.; Haqlwara, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxyanisole in F344 rats234. J. Natl. Cancer Inst. 1983, 70, 343–352. [Google Scholar] [PubMed]
- Roh, J.S.; Lee, H.; Woo, S.; Yoon, M.; Kim, J.; Park, S.D.; Shin, S.S.; Yoon, M. Herbal composition Gambigyeongsinhwan (4) from Curcuma longa, Alnus japonica, and Massa Medicata Fermentata inhibits lipid accumulation in 3T3-L1 cells and regulates obesity in Otsuka Long-Evans Tokushima Fatty rats. J. Ethnopharmacol. 2015, 171, 287–294. [Google Scholar] [PubMed]
- Miranda, P.J.; DeFronzo, R.A.; Califf, R.M.; Guyton, J.R. Metabolic syndrome: Definition, pathophysiology, and mechanisms. Am. Heart J. 2005, 149, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Ma, Y.; Wang, Y.; Du, Z.Y.; Shen, J.K.; Peng, H.L. Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother. Res. 2011, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Seo, M.J.; Choi, H.S.; Lee, B.Y. Pycnogenol® inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother. Res. 2012, 26, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Liu, J.; Klaassen, C.D. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol. Appl. Pharmacol. 2012, 262, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, S.J.; Yang, W.N.; Kuan, A.W.; Chen, C.Y. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro valuations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef]
- Chiang, P.S.; Lee, D.J.; Whiteley, C.G.; Huang, C.Y. Antioxidant phenolic compounds from Pinus morrisconicola using compressional-puffing pretreatment and water-ethanol extraction: Optimization of extraction parameters. J. Taiwan Inst. Chem. Eng. 2017, 70, 7–14. [Google Scholar] [CrossRef]
- Chiang, P.S.; Lee, D.J.; Whiteley, C.G.; Huang, C.Y. Extracting antioxidant phenolic compounds from compressional-puffing pretreated Pinus morrisonicola: Effects of operational parameters, kinetics and characterization. J. Taiwan Inst. Chem. Eng. 2017, 75, 70–76. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Mo, X.F.; Qi, H.M. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hu, N.; Wu, Y.L.; Pan, Y.J.; Sun, C.R. Preliminary studies on the chemical characterization and antioxidant properties of acidic polysaccharides from Sargassum fusiforme. J. Zhejiang Univ. Sci. B 2008, 9, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Tissot, B.; Salpin, J.Y.; Martinez, M.; Gaigeot, M.P.; Daniel, R. Differentiation of the fucoidan sulfated l-fucose isomers constituents by CE-ESIMS and molecular modeling. Carbohydr. Res. 2006, 341, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cui, N.; Bo, Z.X.; Xiang, F.X. Structural determinant and its underlying molecular mechanism of STPC2 related to anti-angiogenic activity. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Skriptsova, A.V.; Zvyagintseva, T.N. Antioxidant activity of fucose-containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Phycol. 2015, 27, 545–553. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.S.; Yu, X.J.; Zhang, Y.Z.; He, R.H.; Ma, H.L. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Zhao, T.T.; Zhang, Q.B.; Qi, H.M.; Zhang, H.; Niu, X.Z.; Xu, Z.H.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.X.; Cao, M.J.; Liu, G.M.; Chen, Q.C.; Sun, L.C.; Chen, H.X. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, Y.; Yang, G.; Yu, L.J. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Yamashita, S.; Sugita-Konishi, Y.; Shimizu, M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001, 7, 262–264. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Karadeniz, F.; Karagozlu, M.Z.; Pyun, S.Y.; Kim, S.K. Sulfation of chitosan oligomers enhances their anti-adipogenic effect in 3T3-L1 adipocytes. Carbohydr. Polym. 2011, 86, 666–671. [Google Scholar] [CrossRef]
- Yoon, S.O.; Kim, M.M.; Park, S.J.; Kim, D.; Chung, J.; Chung, A.S. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J. 2002, 16, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gibbons, M.N. The determination of methylpentoses. Analyst 1955, 80, 268–276. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Athukorala, Y.; Jung, W.K.; Vasanthan, T.; Jeon, Y.J. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr. Polym. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver nanoparticles synthesized by pulsed laser ablation: As a potent antibacterial agent for human enteropathogenic Gram-positive and Gram-negative bacterial strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wu, T.C.; Hsieh, S.L.; Tsai, Y.H.; Yeh, C.W.; Huang, C.Y. Antioxidant activity and growth inhibition of human colon cancer cells by crude and purified fucoidan preparations extracted from Sargassum cristaefolium. J. Food Drug Anal. 2015, 23, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.; Esposito, D.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan seaweeds lower inflammation in RAW 264.7 macrophages and decrease lipid accumulation in 3T3-L1 adipocytes. J. Funct. Foods 2015, 15, 396–407. [Google Scholar] [CrossRef]








| Molecular Weight (MW) | SC | SCO | SCA | SCOA | SCH |
|---|---|---|---|---|---|
| Peak 1 (MW (kDa)/Peak area (%)) | 600.23/100.00 | 608.41/0.41 | 606.54/0.90 | 583.66/0.47 | 645.03/20.11 |
| Peak 2 (MW (kDa)/Peak area (%)) | ND 3 | 10.80/39.61 | 14.89/51.64 | 13.35/70.55 | 22.50/79.89 |
| Peak 3 (MW (kDa)/Peak area (%)) | ND | 5.05/59.98 | 6.87/47.46 | 7.62/28.98 | ND |
| Chemical composition | SC 2 | SCO 2 | SCA 2 | SCOA 2 | SCH 2 |
| Total sugar (%) 1 | 46.51 ± 1.28 b | 45.84 ± 0.81 b | 41.44 ± 2.36 a | 53.17 ± 2.22 c | 50.57 ± 1.51 c |
| Uronic acid (%) 1 | 14.29 ± 0.62 a | 27.71 ± 1.01 c | 27.38 ± 0.94 c | 29.04 ± 1.01 c | 23.71 ± 0.31 b |
| Fucose (%) 1 | 25.87 ± 1.09 a | 24.53 ± 1.89 a | 31.64 ± 1.91 b | 33.20 ± 0.54 b | 30.53 ± 1.89 b |
| Sulfate (%) 1 | 15.12 ± 0.67 a | 17.47 ± 1.35 b | 22.37 ± 0.98 c | 22.23 ± 1.09 c | 19.77 ± 1.01 b |
| Protein (%) 1 | 2.10 ± 0.04 a | 2.08 ± 0.20 a | 2.02 ± 0.01 a | 2.14 ± 0.11 a b | 2.30 ± 0.04 b |
| Polyphenols (%) 1 | 1.01 ± 0.13 c | 1.18 ± 0.06 d | 0.79 ± 0.03 b | 0.62 ± 0.08 a | 0.86 ± 0.06 b |
| Monosaccharide composition (molar ratio) | SC | SCO | SCA | SCOA | SCH |
| Fucose | 1 | 1 | 1 | 1 | 1 |
| Galactose | 0.39 | 0.45 | 0.36 | 0.36 | 0.35 |
| Mannose | 0.30 | 0.36 | 0.38 | 0.34 | 0.34 |
| Glucuronic acid | 0.22 | 0.22 | 0.20 | 0.20 | 0.19 |
| Glucose | 0.15 | 0.10 | 0.10 | 0.09 | 0.09 |
| Rhamnose | 0.20 | 0.10 | 0.04 | 0.05 | 0.05 |
| Xylose | 0.06 | 0.01 | 0.01 | ND | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Kuo, C.-H.; Lee, C.-H. Antibacterial and Antioxidant Capacities and Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by Low-Molecular-Weight Fucoidans Prepared from Compressional-Puffing-Pretreated Sargassum Crassifolium. Mar. Drugs 2018, 16, 24. https://doi.org/10.3390/md16010024
Huang C-Y, Kuo C-H, Lee C-H. Antibacterial and Antioxidant Capacities and Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by Low-Molecular-Weight Fucoidans Prepared from Compressional-Puffing-Pretreated Sargassum Crassifolium. Marine Drugs. 2018; 16(1):24. https://doi.org/10.3390/md16010024
Chicago/Turabian StyleHuang, Chun-Yung, Chia-Hung Kuo, and Chia-Hsin Lee. 2018. "Antibacterial and Antioxidant Capacities and Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by Low-Molecular-Weight Fucoidans Prepared from Compressional-Puffing-Pretreated Sargassum Crassifolium" Marine Drugs 16, no. 1: 24. https://doi.org/10.3390/md16010024
APA StyleHuang, C. -Y., Kuo, C. -H., & Lee, C. -H. (2018). Antibacterial and Antioxidant Capacities and Attenuation of Lipid Accumulation in 3T3-L1 Adipocytes by Low-Molecular-Weight Fucoidans Prepared from Compressional-Puffing-Pretreated Sargassum Crassifolium. Marine Drugs, 16(1), 24. https://doi.org/10.3390/md16010024