Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Animal Material
4.3. Isolation and Purification
4.4. X-ray Crystallographic Analysis
4.5. ECD Calculation
4.6. Antibacterial Assay
4.7. Inhibition of Biofilm Formation Assay
4.8. Confocal Laser Scanning Microscope (CLSM) Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaccari, L.; Allan, D.B.; Sharifi-Mood, N.; Singh, A.R.; Leheny, R.L.; Stebe, K.J. Films of bacteria at interfaces: Three stages of behavior. Soft Matter 2015, 11, 6062–6074. [Google Scholar] [CrossRef] [PubMed]
- Kolter, R. Biofilms in lab and nature: A molecular geneticist’s voyage to microbial ecology. Int. Microbiol. 2010, 13, 1–7. [Google Scholar] [PubMed]
- Koerdt, A.; Godeke, J.; Berger, J.; Thormann, K.M.; Albers, S.J. Crenarchaeal biofilm formation under extreme conditions. PLoS ONE 2010, 5, e14104. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Grenho, L.; Manuel, C.M.; Ferreira, C.; Melo, L.F.; Nunes, O.C.; Monteiro, F.J.; Ferraz, M.P. A modular reactor to simulate biofilm development in orthopedic materials. Int. Microbiol. 2013, 16, 191–198. [Google Scholar] [PubMed]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P. Sponge symbionts play defense. Nat. Chem. Biol. 2014, 10, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Ceratinamides A and B: New antifouling dibromotyrosine derivatives from the marine sponge Pseudoceratina purpurea. Tetrahedron 1996, 52, 8181–8186. [Google Scholar] [CrossRef]
- Shalmali, N.; Ali, M.R.; Bawa, S. Imidazole: An essential edifice for the identification of new lead compounds and drug development. Mini Rev. Med. Chem. 2017, 17. [Google Scholar] [CrossRef]
- Lindel, T. Chemistry and biology of the pyrrole-imidazole alkaloids. Alkaloids Chem. Biol. 2017, 77, 117–219. [Google Scholar] [PubMed]
- Cychon, C.; Lichte, E.; Köck, M. The marine sponge Agelas citrina as a source of the new pyrrole-imidazole alkaloids citrinamines A–D and N-methylagelongine. Beilstein J. Org. Chem. 2015, 11, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Assmann, M.; Soest, R.W.M.; Kock, M. New antifeedant bromopyrrole alkaloid from the Caribbean sponge Stylissa caribica. J. Nat. Prod. 2001, 64, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Liu, H.; Stephens, M.D.; Bewley, C.A.; Melander, C. Marine sponge alkaloids as a source of anti-bacterial adjuvants. Bioorg. Med. Chem. Lett. 2016, 26, 5863–5866. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, Z.; Łos, J.M.; Zula, A.; Zidar, N.; Jakopin, Z.; Łos, M.; Dolenc, M.S.; Ilaš, J.; Wegrzyn, G.; Mašic, L.P.; et al. Inhibition of biofilm formation by conformationally constrained indole-based analogues of the marine alkaloid oroidin. Bioorg. Med. Chem. Lett. 2014, 24, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Forte, B.; Malgesini, B.; Piutti, C.; Quartieri, F.; Scolaro, A.; Papeo, G. A submarine journey: The pyrrole-imidazole alkaloids. Mar. Drugs 2009, 7, 705–753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Khalil, Z.; Conte, M.M.; Plisson, F.; Capon, R.J. A search for kinase inhibitors and antibacterial agents: Bromopyrrolo-2-aminoimidazoles from a deep-water Great Australian Bight sponge, Axinella sp. Tetrahedron Lett. 2012, 53, 3784–3787. [Google Scholar] [CrossRef]
- Autschbach, J.; Nitsch-Velasquez, L.; Rudolph, M. Time-dependent density functional response theory for electronic chiroptical properties of chiral molecules. Top. Curr. Chem. 2011, 298, 1–98. [Google Scholar] [PubMed]
- Pescitelli, G.; Di Pietro, S.; Cardellicchio, C.; Capozzi, M.A.; Di Bari, L. Systematic investigation of CD spectra of aryl benzyl sulfoxides interpreted by means of TDDFT calculations. J. Org. Chem. 2010, 75, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Mauritiamine, a new antifouling oroidin dimer from the marine sponge Agelas mauritiana. J. Nat. Prod. 1996, 59, 501–503. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Amade, P.; Roussakis, C.; Pietra, F. Hanishin, a semiracemic, bioactive C9 alkaloid of the axinellid sponge Acanthella carteri from the Hanish Islands. A shunt metabolite? Tetrahedron Lett. 1997, 38, 6271–6274. [Google Scholar] [CrossRef]
- Forenza, S.; Minale, L.; Riccio, R.; Fattorusso, E. New bromopyrrole derivatives from the sponge Agela soroides. J. Chem. Soc. Chem. Commun. 1971, 18, 1129–1130. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ohizumi, Y.; Nakamura, H.; Hirata, Y. A novel antagonist of serotonergic receptors, hymenidin, isolated from the Okinawan marine sponge Hymeniacidon sp. Experientia 1986, 42, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Cafieri, F.; Fattorusso, E.; Mangoni, A.; Taglialatela-Scafati, O. Dispacamides, anti-histamine alkaloids from caribbean Agelas sponges. Tetrahedron Lett. 1996, 37, 3587–3590. [Google Scholar] [CrossRef]
- Nakamura, H.; Ohizumi, Y.; Kobayashi, J.; Hirata, Y. Keramadine, a novel antagonist of serotonergic receptors isolated from the okinawan sea sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2475–2478. [Google Scholar] [CrossRef]
- Williams, D.E.; Patrick, B.O.; Behrisch, H.W.; Soest, R.V.; Roberge, M.; Andersen, R.J. Dominicin, a cyclic octapeptide, and laughine, a bromopyrrole alkaloid, isolated from the Caribbean marine sponge Eurypon laughlini. J. Nat. Prod. 2005, 68, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Taglialatela-Scafati, O. Two novel pyrrole-imidazole alkaloids from the Mediterranean sponge Agela soroides. Tetrahedron Lett. 2000, 41, 9917–9922. [Google Scholar] [CrossRef]
- Fouad, M.A.; Debbab, A.; Wray, V.; Müller, W.E.G.; Proksch, P. New bioactive alkaloids from the marine sponge Stylissa sp. Tetrahedron 2012, 68, 10176–10179. [Google Scholar] [CrossRef]
- Tasdemir, D.; Mallon, R.; Greenstein, M.; Feldberg, L.R.; Kim, S.C.; Collins, K.; Wojciechowicz, D.; Mangalindan, G.C.; Concepción, G.P.; Harper, M.K.; et al. Aldisine alkaloids from the Philippine sponge Stylissa massa are potent inhibitors of mitogen-activated protein kinase kinase-1 (MEK-1). J. Med. Chem. 2002, 45, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Ohizumi, Y.; Nakamura, H.; Hirata, Y.; Wakamatsu, K.; Miyazawa, T. Hymenin, a novel α-adrenoceptor blocking agent from the Okinawan marine sponge Hymeniacidon sp. Experientia 1986, 42, 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Albizatit, K.F.; Faulkner, D.J. Stevensine, a novel alkaloid of an unidentified marine sponge. J. Org. Chem. 1985, 50, 4163–4164. [Google Scholar] [CrossRef]
- Eder, C.; Proksch, P.; Wray, V.; Klaus Steube, K.; Bringmann, G.; Soest, R.W.M.; Ferdinandus, E.; Pattisina, L.A.; Wiryowidagdo, S.; Moka, W. New alkaloids from the indopacific sponge Stylissa carteri. J. Nat. Prod. 1999, 62, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.W.; Köck, M. NMR Studies of phakellins and isophakellins. J. Nat. Prod. 2008, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Kanda, F.; Ishibashi, M.; Shigemori, H. Manzacidins A–C, novel tetrahydropyrimidine alkaloids from the Okinawan marine sponge Hymeniacidon sp. J. Org. Chem. 1991, 56, 4574–4576. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Tane, K.; Ohta, T.; Matsunaga, S.; Fusetani, N.; Soest, R.W.M. Four new bioactive pyrrole-derived alkaloids from the marine sponge Axinella brevistyla. J. Nat. Prod. 2001, 64, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Gu, B.; Yang, F.; Jiao, W.; Hu, G.; Yu, H.; Han, B.; Zhang, W.; Shen, Y.; et al. Antifungal bromopyrrole alkaloids from the South China Sea sponge Agelas sp. Tetrahedron 2016, 72, 2964–2971. [Google Scholar] [CrossRef]
- Andrade, P.; Willoughby, R.; Pomponi, S.A.; Kerr, R.G. Biosynthetic studies of the alkaloid, stevensine, in a cell culture of the marine sponge Teichaxinella morchella. Tetrahedron Lett. 1999, 40, 4775–4778. [Google Scholar] [CrossRef]
- Stout, E.P.; Wang, Y.; Romo, D.; Molinski, T.F. Pyrrole aminoimidazole alkaloid metabiosynthesis with marine sponges Agelas conifera and Stylissa caribica. Angew. Chem. Int. Ed. 2012, 51, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Genta-Jouve, G.; Cachet, N.; Holderith, S.; Oberhansli, F.; Teyssie, J.L.; Jeffree, R.; Mourabit, A.A.; Thomas, O.P. New insight into marine alkaloid metabolic pathways: Revisiting oroidin biosynthesis. ChemBioChem 2011, 12, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.J.; Reed, C.S.; Christian Melander, C. Effects of N-pyrrole substitution on the anti-biofilm activities of oroidin derivatives against Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2008, 18, 4325–4327. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.E.; Richards, J.J.; Aquino, A.; Reed, C.S.; Melander, C. Antibiofilm activity of a diverse oroidin library generated through reductive acylation. J. Org. Chem. 2009, 74, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Rosenberg, U.; Schneider, J.; Mauerhofer, S.; Maniura-Weber, K.; Ren, Q. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 2016, 100, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Bacterial viability in starved and revitalized biofilms: Comparison of viability staining and direct culture. J. Endod. 2010, 36, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Sauleau, P.; Moriou, C.; Mourabit, A.A. Metabolomics approach to chemical diversity of the Mediterranean marine sponge Agelas oroides. Nat. Prod. Res. 2017, 31, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Pina, I.C.; White, K.N.; Cabrera, G.; Rivero, E.; Crews, P. Bromopyrrole carboxamide biosynthetic products from the Caribbean sponge Agelas dispar. J. Nat. Prod. 2007, 70, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Richelle-Maurer, E.; De Kluijver, M.J.; Feio, S.; Gaudencio, S.; Gaspar, H.; Gomez, R.; Tavares, R.; Van de Vyver, G.; Van Soest, R.W.M. Localization and ecological significance of oroidin and sceptrin in the Caribbean sponge Agelas conifer. Biochem. Syst. Ecol. 2003, 31, 1073–1091. [Google Scholar] [CrossRef]
- Kelly, S.R.; Jensen, P.R.; Henkel, T.P.; Fenical, W.; Pawlik, J.R. Effects of Caribbean sponge extracts on bacterial attachment. Aquat. Microb. Ecol. 2003, 31, 175–182. [Google Scholar] [CrossRef]
- Yamada, A.; Kitamura, H.; Yamaguchi, K.; Fukuzawa, S.; Kamijima, C.; Yazawa, K.; Kuramoto, M.; Wang, G.-Y.-S.; Fujitani, Y.; Uemura, D. Development of chemical substances regulating biofilm formation. Bull. Chem. Soc. Jpn. 1997, 70, 3061–3069. [Google Scholar] [CrossRef]
- Richards, J.J.T.; Ballard, E.B.; Huigens, R.W.; Melander, C. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem 2008, 9, 1267–1279. [Google Scholar] [CrossRef] [PubMed]








| 1 | 2/3 | 4/5 | 6 | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 7.46, d (2.8) | 127.38, CH | 105.20, C | 105.67, C | 6.98, dd (2.4, 2.8) | 121.52, CH | ||
| 2 | 86.79, C | 98.36, C | 99.24, C | 6.52, dd (2.0, 2.4) | 109.32, CH | |||
| 3 | 123.14, C | 6.95, s | 113.46,CH | 6.84, s | 113.86, CH | 119.98, C | ||
| 4 | 102.79, C | 128.36, C | 125.81, C | 126.22, C | ||||
| 5 | 142.18, C | 159.51, C | 157.56, C | 163.29, C | ||||
| 6 | 2.99, m; 2.87, m | 28.42, CH2 | 3.43, m | 42.36, CH2 | 3.77, dd (2.0, 13.20) 3.34, dd (5.2, 13.2) | 42.06, CH2 | 3.48, dd (4.8, 7.2) | 37.60, CH2 |
| 7 | 2.15, m | 21.56, CH2 | 4.37, m | 74.01, CH | 4.64, m | 50.59, CH | 6.84, t (7.2) | 132.54, CH |
| 8 | 4.10, m; 3.98, m | 48.27, CH2 | 5.76, brs | 115.41, CH | 2.63, dd (11.2, 12.8) 2.26, dd (1.5, 12.8) | 36.16, CH2 | 131.59, C | |
| 9 | 152.56, C | 128.58, C | 170.25, C | 165.98, C | ||||
| 10 | 126.39, C | 153.49, C | 4.21, q (7.0) | 60.56, CH2 | ||||
| 11 | 157.51, C | 159.50, C | 1.26, t (7.0) | 14.08, CH3 | ||||
| NH | 12.54, d (2.8) | 12.68, s | 7.87, brd (5.2) | 11.85, dd (2.0, 2.8) | ||||
| NH | 8.15, t (5.0) | 7.73, t (4.8) | ||||||
| NH (CN3H4) | 9.16, s | |||||||
| NH2 (CN3H4) | 7.48, br | 9.23, brs | ||||||
| NH (CN3H4) | 7.24, br | |||||||
| CONH2 | 7.13, brs; 7.54, brs |
| Sample | E. coli Biofilm Formation Inhibition Rate (%) a |
|---|---|
| Positive control b | 98.4 |
| n-BuOH fraction | 7.7 |
| F1 | na |
| F2 | 84.4 |
| F3 | 26.4 |
| F4 | 18.2 |
| F5 | na |
| Sample | E. coli Biofilm Formation Inhibition Rate (%) a | IC50 (μg/mL) |
|---|---|---|
| Positive control | 98.4 ± 0.8 | |
| 12 | 10.4 ± 1.7 | >100 |
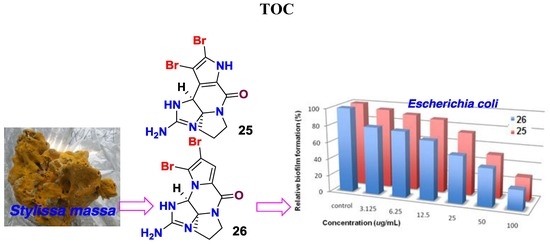
| 25 | 72.1 ± 2.8 | 50.9 |
| 26 | 76.5 ± 1.1 | 31.3 |
| 27 | 25.9 ± 2.4 | >100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wu, J.; An, B.; Voogd, N.J.d.; Cheng, W.; Lin, W. Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria. Mar. Drugs 2018, 16, 9. https://doi.org/10.3390/md16010009
Sun J, Wu J, An B, Voogd NJd, Cheng W, Lin W. Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria. Marine Drugs. 2018; 16(1):9. https://doi.org/10.3390/md16010009
Chicago/Turabian StyleSun, Jingyuan, Jiru Wu, Bang An, Nicole J. de Voogd, Wei Cheng, and Wenhan Lin. 2018. "Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria" Marine Drugs 16, no. 1: 9. https://doi.org/10.3390/md16010009
APA StyleSun, J., Wu, J., An, B., Voogd, N. J. d., Cheng, W., & Lin, W. (2018). Bromopyrrole Alkaloids with the Inhibitory Effects against the Biofilm Formation of Gram Negative Bacteria. Marine Drugs, 16(1), 9. https://doi.org/10.3390/md16010009